当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lewis Acidity and Basicity: Another Measure of Carbene Reactivity.
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpca.0c03877 Trent H Stein 1 , Monica Vasiliu 1 , Anthony J Arduengo 1 , David A Dixon 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jpca.0c03877 Trent H Stein 1 , Monica Vasiliu 1 , Anthony J Arduengo 1 , David A Dixon 1
Affiliation
|
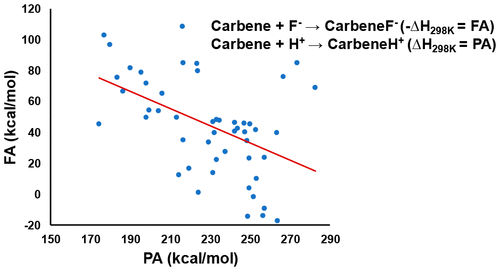
The structural and energetic reactivities of various carbenes are evaluated against a standard electrophile (proton) and a standard nucleophile (fluoride). The proton and fluoride affinities of the carbenes studied provide an increased understanding of reactivity modes and mechanisms. General classification of carbenic reactivity as a singlet nucleophilic carbene or a singlet electrophilic carbene is facilitated by this present study, and a need for further classification means along the border between electrophilic and nucleophilic reactivity is considered. The results are based on electronic structure calculations at the composite correlated molecular orbital theory G3MP2 level.
中文翻译:

Lewis酸度和碱度:衡量碳反应性的另一种方法。
针对标准亲电试剂(质子)和标准亲核试剂(氟化物)评估了各种碳烯的结构和能量反应性。研究的卡宾的质子和氟化物亲和力提供了对反应模式和机理的进一步了解。本研究促进了将羧化反应性分为单线亲核卡宾或单线亲电卡宾的一般分类,并且考虑了沿亲电反应和亲核反应性之间的边界需要进一步分类的手段。结果基于复合相关分子轨道理论G3MP2级别的电子结构计算。
更新日期:2020-07-23
中文翻译:

Lewis酸度和碱度:衡量碳反应性的另一种方法。
针对标准亲电试剂(质子)和标准亲核试剂(氟化物)评估了各种碳烯的结构和能量反应性。研究的卡宾的质子和氟化物亲和力提供了对反应模式和机理的进一步了解。本研究促进了将羧化反应性分为单线亲核卡宾或单线亲电卡宾的一般分类,并且考虑了沿亲电反应和亲核反应性之间的边界需要进一步分类的手段。结果基于复合相关分子轨道理论G3MP2级别的电子结构计算。



























 京公网安备 11010802027423号
京公网安备 11010802027423号