当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal Structure, Polymorphism, and Anisotropic Thermal Expansion of α-Ca(CH3COO)2
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.cgd.0c00563 Sebastian Bette 1, 2, 3 , Gerhard Eggert 2 , Sebastian Emmerling 1, 4 , Martin Etter 5 , Thomas Schleid 3 , Robert E. Dinnebier 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.cgd.0c00563 Sebastian Bette 1, 2, 3 , Gerhard Eggert 2 , Sebastian Emmerling 1, 4 , Martin Etter 5 , Thomas Schleid 3 , Robert E. Dinnebier 1
Affiliation
|
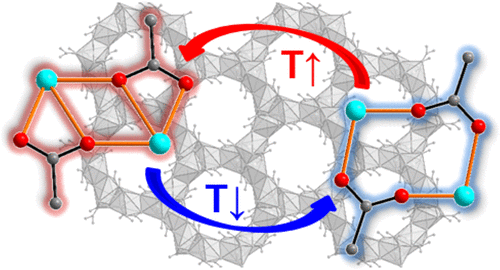
Dehydration of calcium acetate monohydrate (Ca(CH3COO)2·H2O) by heating to 300 °C leads to the formation of anhydrous α-Ca(CH3COO)2. During heating and cooling cycles, high- and low-temperature forms of α-Ca(CH3COO)2 were discovered. The reversible first-order phase transformation between the two forms occurs in a temperature range between 150 and 170 °C. The crystal structures were solved from laboratory powder X-ray diffraction (PXRD) data. The low temperature form of α-calcium acetate (LT-α-Ca(CH3COO)2) crystallizes at room temperature in a primitive triclinic unit cell with space group P1 with lattice parameters of a = 8.7168(3) Å, b = 12.6408(3) Å, c = 12.3084(3) Å, α = 117.4363(17)°, β = 77.827(2)°, γ = 115.053(2)°, and a unit cell volume of 1090.23(6) Å3. High-temperature α-calcium acetate (HT-α-Ca(CH3COO)2) crystallizes at 300 °C in a rhombohedral unit cell with space group R3, lattice parameters of a = 21.1030(5) Å, c = 8.7965(2) Å, and a unit cell volume of 3392.58(17) Å3. In both crystal structures, edge sharing polyhedra of calcium cations and acetate anions that coordinate in both a mono- and bidentate way build up channel-like motifs. During the phase transition, the coordination mode of a bridging acetate anion changes from monodentate to bidentate, and the elliptical channels of the low temperature form become circular. This leads to both negative and positive thermal expansion along different principal axes in the crystal structure of LT-α-Ca(CH3COO)2 and to an overall considerably big volumetric thermal expansion.
中文翻译:

α-Ca(CH 3 COO)2的晶体结构,多态性和各向异性热膨胀
通过加热至300℃使一水合乙酸钙(Ca(CH 3 COO)2 ·H 2 O)脱水导致形成无水α-Ca(CH 3 COO)2。在加热和冷却循环中,发现了高温形式和低温形式的α-Ca(CH 3 COO)2。两种形式之间可逆的一阶相变发生在150至170°C的温度范围内。从实验室粉末X射线衍射(PXRD)数据解析晶体结构。α-乙酸钙(LT-α-Ca(CH 3 COO)2)的低温形式在室温下在具有空间群P的原始三斜晶胞中结晶1的晶格参数为a = 8.7168(3)Å,b = 12.6408(3)Å,c = 12.3084(3)Å,α= 117.4363(17)°,β= 77.827(2)°,γ= 115.053(2 )°,和1090.23(6晶胞体积)埃3。高温α-乙酸钙(HT-α-Ca(CH 3 COO)2)在带有空间群R 3的菱面体晶胞中于300°C结晶,晶格参数a = 21.1030(5)Å,c = 8.7965 (2)埃,和3392.58一个晶胞体积(17)埃3。在这两个晶体结构中,以单齿和双齿方式配位的钙阳离子和乙酸根阴离子的多边共享多面体建立了通道状的图案。在相变过程中,桥联乙酸根阴离子的配位模式从单齿变为双齿,并且低温形式的椭圆形通道变为圆形。这导致沿LT-α-Ca(CH 3 COO)2的晶体结构中的不同主轴方向的负热膨胀和正热膨胀,并且导致整体上相当大的体积热膨胀。
更新日期:2020-08-05
中文翻译:

α-Ca(CH 3 COO)2的晶体结构,多态性和各向异性热膨胀
通过加热至300℃使一水合乙酸钙(Ca(CH 3 COO)2 ·H 2 O)脱水导致形成无水α-Ca(CH 3 COO)2。在加热和冷却循环中,发现了高温形式和低温形式的α-Ca(CH 3 COO)2。两种形式之间可逆的一阶相变发生在150至170°C的温度范围内。从实验室粉末X射线衍射(PXRD)数据解析晶体结构。α-乙酸钙(LT-α-Ca(CH 3 COO)2)的低温形式在室温下在具有空间群P的原始三斜晶胞中结晶1的晶格参数为a = 8.7168(3)Å,b = 12.6408(3)Å,c = 12.3084(3)Å,α= 117.4363(17)°,β= 77.827(2)°,γ= 115.053(2 )°,和1090.23(6晶胞体积)埃3。高温α-乙酸钙(HT-α-Ca(CH 3 COO)2)在带有空间群R 3的菱面体晶胞中于300°C结晶,晶格参数a = 21.1030(5)Å,c = 8.7965 (2)埃,和3392.58一个晶胞体积(17)埃3。在这两个晶体结构中,以单齿和双齿方式配位的钙阳离子和乙酸根阴离子的多边共享多面体建立了通道状的图案。在相变过程中,桥联乙酸根阴离子的配位模式从单齿变为双齿,并且低温形式的椭圆形通道变为圆形。这导致沿LT-α-Ca(CH 3 COO)2的晶体结构中的不同主轴方向的负热膨胀和正热膨胀,并且导致整体上相当大的体积热膨胀。



























 京公网安备 11010802027423号
京公网安备 11010802027423号