当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ni‐ and Co‐Substituted Metallic MoS2 for the Alkaline Hydrogen Evolution Reaction
ChemElectroChem ( IF 4 ) Pub Date : 2020-06-11 , DOI: 10.1002/celc.202000532 Nuwan H. Attanayake 1, 2 , Lakshay Dheer 3, 4, 5 , Akila C. Thenuwara 1, 2 , Sasitha C. Abeyweera 1 , Coby Collins 1 , Umesh V. Waghmare 3, 4 , Daniel R. Strongin 1, 2
ChemElectroChem ( IF 4 ) Pub Date : 2020-06-11 , DOI: 10.1002/celc.202000532 Nuwan H. Attanayake 1, 2 , Lakshay Dheer 3, 4, 5 , Akila C. Thenuwara 1, 2 , Sasitha C. Abeyweera 1 , Coby Collins 1 , Umesh V. Waghmare 3, 4 , Daniel R. Strongin 1, 2
Affiliation
|
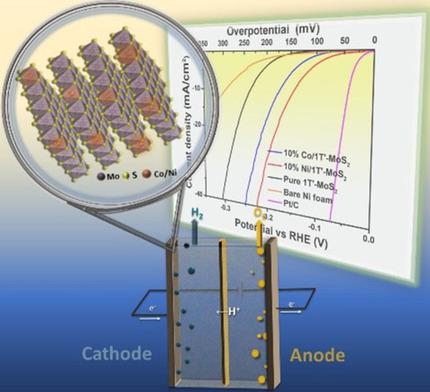
A metallic MoS2 (M−MoS2) catalyst containing either Ni or Co with excellent activity for the hydrogen evolution reaction (HER) under alkaline electrocatalytic conditions was investigated. To synthesize the 3d transition metal containing electrocatalysts, 1–20 at.% Ni or Co was substituted into the lattice of orthorhombic MoO3 and the doped metal oxide precursor was sulfided and converted to Ni/M−MoS2 or Co/M−MoS2. Raman spectroscopy and photoelectron spectroscopy were used to verify that the Ni or Co substituted MoS2 was the metallic‐like 1T′ phase of the metal dichalcogenide. The 10 at.% Ni/M−MoS2 (10 at.% Co/M−MoS2) electrochemical HER catalyst investigated under alkaline conditions exhibited a low onset potential of ∼−75 mV (∼−100 mV) and overpotential of −145 mV (−160 mV) at a current density of 10 mA/cm2. Pristine M−MoS2 exhibited both a higher onset potential of ∼−150 mV and a higher overpotential of −240 mV at 10 mA/cm2. First‐principles density functional theory analysis showed that substitution of 3d transition metals like Ni and Co in the metallic MoS2 structure notably stabilized the distorted polytype of 1T′−MoS2, lowering the free energy for H binding and H2O dissociation, both important steps in alkaline HER, giving rise to enhanced activity towards HER.
中文翻译:

镍和共取代的金属MoS2用于碱性氢释放反应
研究了含有Ni或Co的金属MoS 2(M-MoS 2)催化剂在碱性电催化条件下对氢释放反应(HER)具有优异的活性。为了合成含3d过渡金属的电催化剂,将1-20 at。%的Ni或Co代入斜方MoO 3的晶格中,然后将掺杂的金属氧化物前驱体硫化并转化为Ni / M-MoS 2或Co / M-MoS 2。拉曼光谱法和光电子能谱法用于验证Ni或Co取代的MoS 2是金属二卤化物的金属样1T'相。10 at。%Ni / M-MoS 2(10 at。%Co / M-MoS 2)在碱性条件下研究的电化学HER催化剂在10 mA / cm 2的电流密度下显示出低的起始电势-75 mV(--100 mV)和过电势-145 mV(-160 mV)。原始M-MoS 2在10 mA / cm 2时显示出较高的起始电位〜-150 mV和较高的过电位-240 mV 。一原理密度泛函理论分析表明,在金属MoS 2结构中置换3d过渡金属(如Ni和Co)显着稳定了扭曲的1T'-MoS 2多型,降低了H结合和H 2 O离解的自由能,两者碱性HER的重要步骤,从而增强了对HER的活性。
更新日期:2020-06-11
中文翻译:

镍和共取代的金属MoS2用于碱性氢释放反应
研究了含有Ni或Co的金属MoS 2(M-MoS 2)催化剂在碱性电催化条件下对氢释放反应(HER)具有优异的活性。为了合成含3d过渡金属的电催化剂,将1-20 at。%的Ni或Co代入斜方MoO 3的晶格中,然后将掺杂的金属氧化物前驱体硫化并转化为Ni / M-MoS 2或Co / M-MoS 2。拉曼光谱法和光电子能谱法用于验证Ni或Co取代的MoS 2是金属二卤化物的金属样1T'相。10 at。%Ni / M-MoS 2(10 at。%Co / M-MoS 2)在碱性条件下研究的电化学HER催化剂在10 mA / cm 2的电流密度下显示出低的起始电势-75 mV(--100 mV)和过电势-145 mV(-160 mV)。原始M-MoS 2在10 mA / cm 2时显示出较高的起始电位〜-150 mV和较高的过电位-240 mV 。一原理密度泛函理论分析表明,在金属MoS 2结构中置换3d过渡金属(如Ni和Co)显着稳定了扭曲的1T'-MoS 2多型,降低了H结合和H 2 O离解的自由能,两者碱性HER的重要步骤,从而增强了对HER的活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号