Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.tetlet.2020.152098 Aman Bhalla , Garima Modi , Pooja Yadav , Pankaj Kumar , S.S. Bari , Geeta Hundal
|
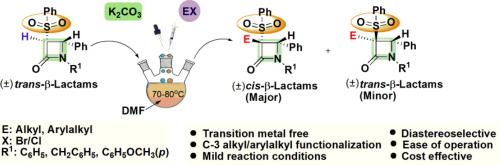
A sulfonyl promoted synthetic protocol for the C-3 alkylation of trans-3-phenylsulfonyl-β-lactams 6(a-e) with active organic halides in presence of K2CO3 as mild base and DMF as solvent is described. This protocol furnished cis- and trans-β-lactams as major and minor isomers respectively with alkyl halides while arylalkyl/unsaturated halides yield only cis-β-lactams exclusively. Further, the effect of sterically bulky group on the C-3 substitution was investigated by the reaction of 3-phenylsulfonyl-β-lactams 6(d-e) with crotyl chloride (predominantly E) to achieve diastereomeric mixture of β-lactams 7/7ʹ and 8. This strategy reveals advantages in terms of cost effectiveness, functional group tolerance and ease of operation.
中文翻译:

反式3-苯基磺酰基-β-内酰胺与有机卤化物的立体选择性C-3烷基化反应,使用磺酰基部分作为活化基团来获得C-3取代的β-内酰胺
描述了在以K 2 CO 3为弱碱和以DMF为溶剂的条件下,用活性有机卤化物对反式-3-苯基磺酰基-β-内酰胺6(ae)进行C-3烷基化的磺酰基促进合成方案。该方案分别将顺式和反式-β-内酰胺作为主要和次要异构体提供了烷基卤,而芳基烷基/不饱和卤化物仅仅产生顺式-β-内酰胺。此外,通过使3-苯基磺酰基-β-内酰胺6(de)与巴豆酰氯(主要为E)反应,获得β-内酰胺的非对映异构混合物,研究了空间大基团对C-3取代的影响。7 / 7ʹ和8。该策略在成本效益,功能组容忍度和操作简便性方面显示出优势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号