Reactive & Functional Polymers ( IF 5.1 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.reactfunctpolym.2020.104675 Yuliang Jiang , Mostafa R. Abukhadra , Nermen M. Refay , Mohamed F. Sharaf , Mohammed A. El-Meligy , Emad Mahrous Awwad
|
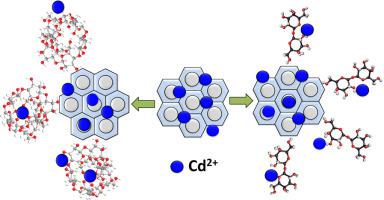
Chitosan/MCM-48 (CH/MCM) and β-cyclodextrin/MCM-48 (CD/MCM) composites were synthesized as promising and eco-friendly bio-adsorbents for Cd2+ ions from water. The composites showed novel porous structures and complex functional groups making them of high adsorption capacities. The adsorption behaviors of CH/MCM and CD/MCM are highly controlled by the tested pH values realizing their best capacities at pH 7. The kinetic evaluation indicated the excellent agreement between the uptake of Cd2+ by CH/MCM and CD/MCM with the pseudo-first-order model achieving equilibrium intervals of 480 min and 600 min, respectively. Based on the values of Chi-squared (X2) and the correlation coefficient, the composites displayed adsorption properties related to the Freundlich hypothesis with a multilayer form. Additionally, the Gaussian energies of them are 2.23 KJ/mol (CH/MCM) and 2.46 KJ/mol (CD/MCM) reflecting physical uptake of Cd2+ by the studied composites. The thermodynamic investigation implied spontaneous uptake of Cd2+ by the composites with endothermic reactions. The prepared CH/MCM and CD/MCM are of 122.4 mg/g and 152.2 mg/g theoretical qmax, respectively which are higher values than the reported results for several studied adsorbents. Moreover, the synthetic CH/MCM and CD/MCM showed high reusability as adsorbents for Cd2+ to be applied effectively six times.
中文翻译:

壳聚糖/ MCM-48和β-环糊精/ MCM-48复合材料的合成作为环境吸附Cd 2+离子的生物吸附剂;动力学和平衡研究
壳聚糖/ MCM-48(CH / MCM)和β-环糊精/ MCM-48(CD / MCM)复合材料被合成为有前途的,对水中Cd 2+离子具有生态友好性的生物吸附剂。该复合材料显示出新颖的多孔结构和复杂的官能团,使其具有高吸附能力。CH / MCM和CD / MCM的吸附行为受到所测试的pH值的严格控制,从而在pH 7时实现了最佳容量。动力学评估表明,CH / MCM和CD / MCM吸收Cd 2+与CD / MCM之间的优异一致性伪一阶模型分别实现了480分钟和600分钟的平衡间隔。基于卡方(X 2)和相关系数,该复合材料表现出与Freundlich假设有关的多层吸附性能。此外,它们的高斯能量分别为2.23 KJ / mol(CH / MCM)和2.46 KJ / mol(CD / MCM),反映了所研究复合材料对Cd 2+的物理吸收。热力学研究表明,具有吸热反应的复合材料会自发吸收Cd 2+。所制备的CH / MCM和CD / MCM的理论q max分别为122.4 mg / g和152.2 mg / g ,高于几种研究的吸附剂的报告结果。而且,合成的CH / MCM和CD / MCM作为有效吸附六次的Cd 2+吸附剂具有很高的可重复使用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号