Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.bmcl.2020.127350 Ana C A de Souza 1 , Mattia Mori 2 , Larissa Sens 3 , Ruth F Rocha 1 , Tiago Tizziani 3 , Luiz F S de Souza 3 , Louise Domeneghini Chiaradia-Delatorre 3 , Maurizio Botta 2 , Ricardo J Nunes 3 , Hernán Terenzi 1 , Angela C O Menegatti 4
|
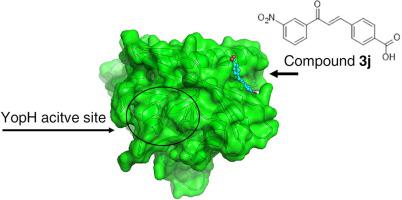
Identification of allosteric inhibitors of PTPs has attracted great interest as a new strategy to overcome the challenge of discover potent and selective molecules for therapeutic intervention. YopH is a virulence factor of the genus Yersinia, validated as an antimicrobial target. The finding of a second substrate binding site in YopH has revealed a putative allosteric site that could be further exploited. Novel chalcone compounds that inhibit PTPs activity were designed and synthesized. Compound 3j was the most potent inhibitor, interestingly, with different mechanisms of inhibition for the panel of enzymes evaluated. Further, our results showed that compound 3j is an irreversible non-competitive inhibitor of YopH that binds to a site different than the catalytic site, but close to the well-known second binding site of YopH.
中文翻译:

查尔酮衍生物与YopH的假定变构位点结合:抑制耶尔森氏菌的致病因子。
PTP的别构抑制剂的鉴定作为一种新的策略已引起了极大的兴趣,该新策略可克服发现有效和选择性分子进行治疗干预的挑战。YopH是耶尔森氏菌属的一种毒力因子,已被确认为抗微生物靶标。YopH中第二个底物结合位点的发现揭示了一个推测的变构位点,可以进一步利用它。设计并合成了抑制PTP活性的新型查尔酮化合物。有趣的是,化合物3j是最有效的抑制剂,对所评估的酶具有不同的抑制机制。此外,我们的结果表明化合物3j 是YopH的不可逆的非竞争性抑制剂,它与不同于催化位点但与著名的YopH第二结合位点结合的位点结合。


























 京公网安备 11010802027423号
京公网安备 11010802027423号