当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of C-type lectin-oriented surfaces for high avidity glycoconjugates: towards mimicking multivalent interactions on the cell surface.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0ob00781a Vanessa Porkolab 1 , Carlo Pifferi 2 , Ieva Sutkeviciute 1 , Stefania Ordanini 3 , Marwa Taouai 4 , Michel Thépaut 1 , Corinne Vivès 1 , Mohammed Benazza 4 , Anna Bernardi 3 , Olivier Renaudet 2 , Franck Fieschi 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0ob00781a Vanessa Porkolab 1 , Carlo Pifferi 2 , Ieva Sutkeviciute 1 , Stefania Ordanini 3 , Marwa Taouai 4 , Michel Thépaut 1 , Corinne Vivès 1 , Mohammed Benazza 4 , Anna Bernardi 3 , Olivier Renaudet 2 , Franck Fieschi 1
Affiliation
|
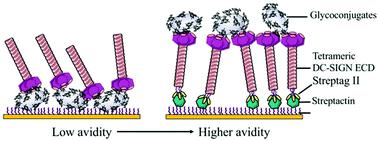
Multivalent interactions between complex carbohydrates and oligomeric C-type lectins govern a wide range of immune responses. Up to date, standard SPR (surface plasmon resonance) competitive assays have largely been to evaluate binding properties from monosaccharide units (low affinity, mM) to multivalent elemental antagonists (moderate affinity, μM). Herein, we report typical case-studies of SPR competitive assays showing that they underestimate the potency of glycoclusters to inhibit the interaction between DC-SIGN and immobilized glycoconjugates. This paper describes the design and implementation of a SPR direct interaction over DC-SIGN oriented surfaces, extendable to other C-type lectin surfaces as such Langerin. This setup provides an overview of intrinsic avidity generation emanating simultaneously from multivalent glycoclusters and from DC-SIGN tetramers organized in nanoclusters at the cell membrane. To do so, covalent biospecific capture of DC-SIGN via StreptagII/StrepTactin interaction preserves tetrameric DC-SIGN, accessibility and topology of its active sites, that would have been dissociated using standard EDC–NHS procedure under acidic conditions. From the tested glycoclusters libraries, we demonstrated that the scaffold architecture, the valency and the glycomimetic-based ligand are crucial to reach nanomolar affinities for DC-SIGN. The glycocluster 3·D illustrates the tightest binding partner in this set for a DC-SIGN surface (KD = 18 nM). Moreover, the selectivity at monovalent scale of glycomimetic D can be easily analyzed at multivalent scale comparing its binding over different C-type lectin immobilized surfaces. This approach may give rise to novel insights into the multivalent binding mechanisms responsible for avidity and make a major contribution to the full characterization of the binding potency of promising specific and multivalent immodulators.
中文翻译:

用于高亲和力糖缀合物的C型凝集素定向表面的开发:模仿细胞表面上的多价相互作用。
复杂碳水化合物与寡聚C型凝集素之间的多价相互作用控制着广泛的免疫反应。迄今为止,标准的SPR(表面等离振子共振)竞争测定法主要用于评估从单糖单位(低亲和力,mM)到多价元素拮抗剂(中等亲和力,μM)的结合特性。在本文中,我们报告了SPR竞争测定的典型案例研究,显示它们低估了糖簇抑制DC-SIGN和固定化糖缀合物之间相互作用的能力。本文介绍了在面向DC-SIGN的表面上SPR直接相互作用的设计和实现,该表面可扩展到其他C型凝集素表面,例如Langerin。此设置概述了同时发生的内在亲和力生成,这些内在亲和力生成是同时从多价糖簇和组织在细胞膜上的纳米簇中的DC-SIGN四聚体发出的。为此,共价捕获DC-SIGN的生物特异性通过StreptagII / StrepTactin相互作用可以保留四聚体DC-SIGN,其活性位点的可及性和拓扑结构,而在酸性条件下使用标准EDC-NHS程序可以将其分离。从测试的糖簇库中,我们证明了支架结构,化合价和基于糖模拟物的配体对于达到DC-SIGN的纳摩尔亲和力至关重要。糖簇3·D说明了该组中对于DC-SIGN表面最紧密的结合伴侣(K D= 18nM)。此外,拟糖D的单价选择性可以容易地在多价规模上进行比较,比较其在不同C型凝集素固定化表面上的结合。该方法可能引起对负责亲合力的多价结合机制的新颖见解,并且对有前途的特异性和多价调节子的结合能力的全面表征做出了重大贡献。
更新日期:2020-07-01
中文翻译:

用于高亲和力糖缀合物的C型凝集素定向表面的开发:模仿细胞表面上的多价相互作用。
复杂碳水化合物与寡聚C型凝集素之间的多价相互作用控制着广泛的免疫反应。迄今为止,标准的SPR(表面等离振子共振)竞争测定法主要用于评估从单糖单位(低亲和力,mM)到多价元素拮抗剂(中等亲和力,μM)的结合特性。在本文中,我们报告了SPR竞争测定的典型案例研究,显示它们低估了糖簇抑制DC-SIGN和固定化糖缀合物之间相互作用的能力。本文介绍了在面向DC-SIGN的表面上SPR直接相互作用的设计和实现,该表面可扩展到其他C型凝集素表面,例如Langerin。此设置概述了同时发生的内在亲和力生成,这些内在亲和力生成是同时从多价糖簇和组织在细胞膜上的纳米簇中的DC-SIGN四聚体发出的。为此,共价捕获DC-SIGN的生物特异性通过StreptagII / StrepTactin相互作用可以保留四聚体DC-SIGN,其活性位点的可及性和拓扑结构,而在酸性条件下使用标准EDC-NHS程序可以将其分离。从测试的糖簇库中,我们证明了支架结构,化合价和基于糖模拟物的配体对于达到DC-SIGN的纳摩尔亲和力至关重要。糖簇3·D说明了该组中对于DC-SIGN表面最紧密的结合伴侣(K D= 18nM)。此外,拟糖D的单价选择性可以容易地在多价规模上进行比较,比较其在不同C型凝集素固定化表面上的结合。该方法可能引起对负责亲合力的多价结合机制的新颖见解,并且对有前途的特异性和多价调节子的结合能力的全面表征做出了重大贡献。


























 京公网安备 11010802027423号
京公网安备 11010802027423号