Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electroactivation-induced IrNi nanoparticles under different pH conditions for neutral water oxidation.
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0nr02951c Woong Hee Lee 1 , Jaekyung Yi , Hong Nhan Nong , Peter Strasser , Keun Hwa Chae , Byoung Koun Min , Yun Jeong Hwang , Hyung-Suk Oh
Nanoscale ( IF 6.7 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0nr02951c Woong Hee Lee 1 , Jaekyung Yi , Hong Nhan Nong , Peter Strasser , Keun Hwa Chae , Byoung Koun Min , Yun Jeong Hwang , Hyung-Suk Oh
Affiliation
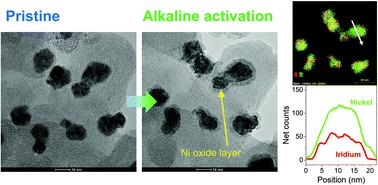
|
Electrochemical oxidation processes can affect the electronic structure and activate the catalytic performance of precious-metal and transition-metal based catalysts for the oxygen evolution reaction (OER). Also there are emerging requirements to develop OER electrocatalysts under various pH conditions in order to couple with different reduction reactions. Herein, we studied the effect of pH on the electroactivation of IrNi alloy nanoparticles supported on carbon (IrNi/C) and evaluated the electrocatalytic activities of the activated IrNiOx/C for water oxidation under neutral conditions. In addition, their electronic structures and atomic arrangement were analyzed by in situ/operando X-ray absorption spectroscopy (XAS) and identical location transmission electron microscopy techniques, showing the reconstruction of the metal elements during electroactivation due to their different stabilities depending on the electrolyte pH. IrNiOx/C activated under neutral pH conditions showed a mildly oxidized thin IrOx shell. Meanwhile, IrNiOx/C activated in acidic and alkaline electrolytes showed Ni-leached IrOx and Ni-rich IrNiOx surfaces, respectively. Particularly, the surface of IrNiOx/C activated under alkaline conditions shows IrOx with a high d-band hole and NiOx with a high oxidation state leading to excellent OER catalytic activity in neutral media (η = 384 mV at 10 mA cm−2) whereas much lower OER activity was reported under alkaline or acid conditions. Our results, which showed that electrochemically activated catalysts under different pH conditions exhibit a unique electronic structure by modifying the initial alloy catalyst, can be applied for the design of catalysts suitable for various electrochemical reactions.
中文翻译:

电活化诱导的IrNi纳米粒子在不同pH条件下用于中性水氧化。
电化学氧化过程会影响电子结构,并激活基于贵金属和过渡金属的催化剂对氧气释放反应(OER)的催化性能。还出现了在各种pH条件下开发OER电催化剂以结合不同的还原反应的新要求。本文中,我们研究了pH值对负载在碳上的IrNi合金纳米颗粒(IrNi / C)的电活化的影响,并评估了中性条件下活化的IrNiO x / C对水氧化的电催化活性。此外,通过原位/操作分析了它们的电子结构和原子排列。X射线吸收光谱法(XAS)和相同的位置透射电子显微镜技术,显示了金属元素在电活化过程中的重构,这是由于其取决于电解质pH的不同稳定性。在中性pH条件下活化的IrNiO x / C显示出轻度氧化的薄IrO x壳。同时,在酸性和碱性电解质中活化的IrNiO x / C分别显示出Ni浸出的IrO x和富Ni的IrNiO x表面。特别地,在碱性条件下活化的IrNiO x / C的表面显示出具有高d带孔的IrO x和NiO x具有较高的氧化态,导致在中性介质(10 mA cm -2时η = 384 mV )下具有出色的OER催化活性,而在碱性或酸性条件下,则报告的OER活性要低得多。我们的结果表明,通过改变初始合金催化剂,在不同pH条件下的电化学活化催化剂表现出独特的电子结构,可用于设计适合各种电化学反应的催化剂。
更新日期:2020-07-16
中文翻译:

电活化诱导的IrNi纳米粒子在不同pH条件下用于中性水氧化。
电化学氧化过程会影响电子结构,并激活基于贵金属和过渡金属的催化剂对氧气释放反应(OER)的催化性能。还出现了在各种pH条件下开发OER电催化剂以结合不同的还原反应的新要求。本文中,我们研究了pH值对负载在碳上的IrNi合金纳米颗粒(IrNi / C)的电活化的影响,并评估了中性条件下活化的IrNiO x / C对水氧化的电催化活性。此外,通过原位/操作分析了它们的电子结构和原子排列。X射线吸收光谱法(XAS)和相同的位置透射电子显微镜技术,显示了金属元素在电活化过程中的重构,这是由于其取决于电解质pH的不同稳定性。在中性pH条件下活化的IrNiO x / C显示出轻度氧化的薄IrO x壳。同时,在酸性和碱性电解质中活化的IrNiO x / C分别显示出Ni浸出的IrO x和富Ni的IrNiO x表面。特别地,在碱性条件下活化的IrNiO x / C的表面显示出具有高d带孔的IrO x和NiO x具有较高的氧化态,导致在中性介质(10 mA cm -2时η = 384 mV )下具有出色的OER催化活性,而在碱性或酸性条件下,则报告的OER活性要低得多。我们的结果表明,通过改变初始合金催化剂,在不同pH条件下的电化学活化催化剂表现出独特的电子结构,可用于设计适合各种电化学反应的催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号