当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protonic acid doping of low band-gap conjugated polyions
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0qm00278j Gang Ye 1, 2, 3, 4, 5 , Yuru Liu 1, 2, 3, 4, 5 , Jian Liu 3, 4, 5, 6 , Xinkai Qiu 1, 2, 3, 4, 5 , L. Jan Anton Koster 3, 4, 5, 6 , Ryan C. Chiechi 1, 2, 3, 4, 5
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2020-06-11 , DOI: 10.1039/d0qm00278j Gang Ye 1, 2, 3, 4, 5 , Yuru Liu 1, 2, 3, 4, 5 , Jian Liu 3, 4, 5, 6 , Xinkai Qiu 1, 2, 3, 4, 5 , L. Jan Anton Koster 3, 4, 5, 6 , Ryan C. Chiechi 1, 2, 3, 4, 5
Affiliation
|
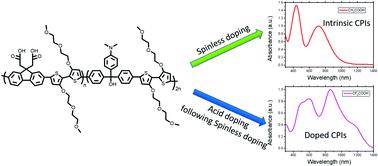
This paper describes the design and synthesis of a series of conjugated polyions (CPIZ-T, CPIZ-TT and CPIZ-TT-DEG) that incorporate a formal positive charge into their conjugated backbones, balanced by anionic pendant groups with increasing electron-donating ability. The energy levels and the bandgap of these conjugated polyions were determined by using optical absorption spectroscopy and cyclic voltammetry (CV) and were easily modulated by varying the electron donating group. The energies of the occupied states increase with increasing electron-donating ability, while the energies of the unoccupied states are almost unchanged due to the presence of tritylium ions in the conjugated backbone. All conjugated polyions exhibit pristine semiconducting properties in weak protonic acids, but with sufficiently strong acids, the polymers exhibit spontaneous spin unpairing and convert to a metallic state. The required strength of the acids varies with the electron-donating ability, with higher HOMO levels leading to more facile proton acid doping and higher electrical conductivities. The mechanism of protonic acid doping of conjugated polyions involves a spinless doping process (dehydration) followed by a spontaneous spin unpairing leading to the formation of polarons. While protonic acid doping occurs in polyaniline, conjugated polyions offer synthetic tunability and selective processing into insulating, semiconducting and metallic states simply by controlling acidity.
中文翻译:

低带隙共轭聚离子的质子酸掺杂
本文介绍了一系列共轭聚离子(CPIZ-T,CPIZ-TT和CPIZ-TT-DEG)的设计与合成。),将形式上的正电荷结合到其共轭主链中,并通过阴离子侧基增加了供电子能力。这些共轭聚离子的能级和带隙是通过使用光吸收光谱法和循环伏安法(CV)来确定的,并且可以通过改变供电子基团来轻松地进行调节。占据态的能量随供电子能力的增加而增加,而未占据态的能量由于共轭主链中存在tr离子而几乎未变。所有共轭聚离子在弱质子酸中均表现出原始的半导体性能,但是在足够强的酸中,聚合物表现出自发的自旋不成对并转化为金属态。酸的所需强度随供电子能力而变化,较高的HOMO含量导致更容易的质子酸掺杂和较高的电导率。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。
更新日期:2020-06-24
中文翻译:

低带隙共轭聚离子的质子酸掺杂
本文介绍了一系列共轭聚离子(CPIZ-T,CPIZ-TT和CPIZ-TT-DEG)的设计与合成。),将形式上的正电荷结合到其共轭主链中,并通过阴离子侧基增加了供电子能力。这些共轭聚离子的能级和带隙是通过使用光吸收光谱法和循环伏安法(CV)来确定的,并且可以通过改变供电子基团来轻松地进行调节。占据态的能量随供电子能力的增加而增加,而未占据态的能量由于共轭主链中存在tr离子而几乎未变。所有共轭聚离子在弱质子酸中均表现出原始的半导体性能,但是在足够强的酸中,聚合物表现出自发的自旋不成对并转化为金属态。酸的所需强度随供电子能力而变化,较高的HOMO含量导致更容易的质子酸掺杂和较高的电导率。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。共轭聚离子的质子酸掺杂机理涉及无旋掺杂过程(脱水),然后是自发的自旋不成对,从而导致极化子的形成。尽管质子酸掺杂发生在聚苯胺中,但共轭聚离子仅通过控制酸度即可提供合成可调性和选择性加工成绝缘状态,半导体状态和金属状态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号