当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process-Controlled Regiodivergent Copper-Catalyzed Azide-Alkyne Cycloadditions: Tailor-made Syntheses of 4- and 5-Bromotriazoles from Bromo(phosphoryl)ethyne.
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.orglett.0c01681 Yasuhiro Okuda , Kazuto Imafuku , Yoshiyuki Tsuchida , Tomoyo Seo , Haruo Akashi , Akihiro Orita
Organic Letters ( IF 5.2 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.orglett.0c01681 Yasuhiro Okuda , Kazuto Imafuku , Yoshiyuki Tsuchida , Tomoyo Seo , Haruo Akashi , Akihiro Orita
|
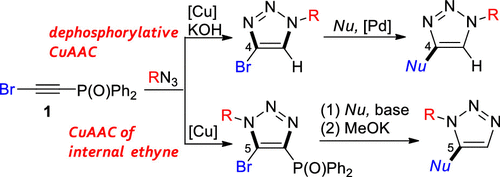
We developed a regiodivergent syntheses of 4- and 5-bromo-substituted 1,2,3-triazoles in copper-catalyzed azide–alkyne cycloadditions (CuAACs) by taking advantage of bromo(phosphoryl)ethyne 1 as a bromoethyne equivalent. A one-shot dephosphorylative CuAAC of 1 afforded 4-bromotriazoles, which was transformed into a histone deacetylase 8 (HDAC8)-selective inhibitor, NCC-149. However, the direct CuAAC catalyzed by CuI/Cu(OAc)2 provided 5-bromo-4-phosphoryltriazoles. The consecutive nucleophilic substitution of the bromo group with thiols followed by MeOK-promoted dephosphorylation gave 5-thio-substituted triazoles.
中文翻译:

过程控制的区域发散性铜催化的叠氮化物-炔烃环加成反应:量身定制的由溴(磷酰基)乙炔合成的4-和5-溴三唑。
我们通过利用溴(磷酰基)乙炔1作为溴乙炔当量的优势,在铜催化的叠氮化物-炔烃环加成反应(CuAAC)中开发了4-和5-溴取代的1,2,3-三唑的区域发散性合成。一次性脱磷酸化CuAAC为1可得到4-溴三唑,将其转化为组蛋白脱乙酰基酶8(HDAC8)选择性抑制剂NCC-149。然而,由CuI / Cu(OAc)2催化的直接CuAAC提供了5-溴-4-磷酰基三唑。溴基团被硫醇连续亲核取代,然后由MeOK促进的去磷酸化,得到5-硫基取代的三唑。
更新日期:2020-07-02
中文翻译:

过程控制的区域发散性铜催化的叠氮化物-炔烃环加成反应:量身定制的由溴(磷酰基)乙炔合成的4-和5-溴三唑。
我们通过利用溴(磷酰基)乙炔1作为溴乙炔当量的优势,在铜催化的叠氮化物-炔烃环加成反应(CuAAC)中开发了4-和5-溴取代的1,2,3-三唑的区域发散性合成。一次性脱磷酸化CuAAC为1可得到4-溴三唑,将其转化为组蛋白脱乙酰基酶8(HDAC8)选择性抑制剂NCC-149。然而,由CuI / Cu(OAc)2催化的直接CuAAC提供了5-溴-4-磷酰基三唑。溴基团被硫醇连续亲核取代,然后由MeOK促进的去磷酸化,得到5-硫基取代的三唑。


























 京公网安备 11010802027423号
京公网安备 11010802027423号