当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Determinants of the Dopamine Transporter Regulation Mediated by G Proteins.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jcim.0c00236 Genoveva Rojas 1 , Ivana Orellana 1 , Roberto Rosales-Rojas 1 , Jennie García-Olivares 2 , Jeffrey Comer 3 , Ariela Vergara-Jaque 1, 4
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-06-11 , DOI: 10.1021/acs.jcim.0c00236 Genoveva Rojas 1 , Ivana Orellana 1 , Roberto Rosales-Rojas 1 , Jennie García-Olivares 2 , Jeffrey Comer 3 , Ariela Vergara-Jaque 1, 4
Affiliation
|
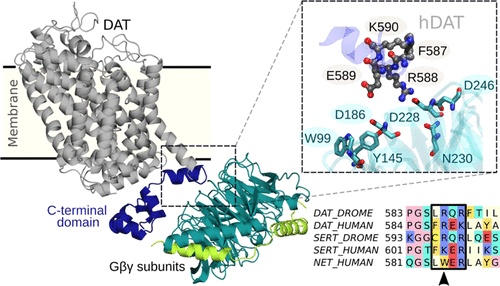
Dopamine clearance in the brain is controlled by the dopamine transporter (DAT), a protein residing in the plasma membrane, which drives reuptake of extracellular dopamine into presynaptic neurons. Studies have revealed that the βγ subunits of heterotrimeric G proteins modulate DAT function through a physical association with the C-terminal region of the transporter. Regulation of neurotransmitter transporters by Gβγ subunits is unprecedented in the literature; therefore, it is interesting to investigate the structural details of this particular protein–protein interaction. Here, we refined the crystal structure of the Drosophila melanogaster DAT (dDAT), modeling de novo the N- and C-terminal domains; subsequently, we used the full-length dDAT structure to generate a comparative model of human DAT (hDAT). Both proteins were assembled with Gβ1γ2 subunits employing protein–protein docking, and subsequent molecular dynamics simulations were run to identify the specific interactions governing the formation of the hDAT:Gβγ and dDAT:Gβγ complexes. A [L/F]R[Q/E]R sequence motif containing the residues R588 in hDAT and R587 in dDAT was found as key to bind the Gβγ subunits through electrostatic interactions with a cluster of negatively charged residues located at the top face of the Gβ subunit. Alterations of DAT function have been associated with multiple devastating neuropathological conditions; therefore, this work represents a step toward better understanding DAT regulation by signaling proteins, allowing us to predict therapeutic target regions.
中文翻译:

G 蛋白介导的多巴胺转运蛋白调控的结构决定因素。
大脑中的多巴胺清除由多巴胺转运蛋白 (DAT) 控制,DAT 是一种位于质膜中的蛋白质,可驱动细胞外多巴胺再摄取到突触前神经元中。研究表明,异源三聚体 G 蛋白的 βγ 亚基通过与转运蛋白 C 末端区域的物理关联来调节 DAT 功能。Gβγ亚基对神经递质转运蛋白的调节在文献中是前所未有的。因此,研究这种特定蛋白质-蛋白质相互作用的结构细节很有趣。在这里,我们改进了黑腹果蝇DAT (dDAT)的晶体结构,从头建模N-和C-末端结构域;随后,我们使用全长 dDAT 结构生成人类 DAT (hDAT) 的比较模型。两种蛋白质均采用蛋白质-蛋白质对接与 Gβ1γ2 亚基组装,随后进行分子动力学模拟以确定控制 hDAT:Gβγ 和 dDAT:Gβγ 复合物形成的特定相互作用。发现包含 hDAT 中残基 R588 和 dDAT 中 R587 的 [L/F]R[Q/E]R 序列基序是通过与位于顶部的带负电荷残基簇的静电相互作用结合 Gβγ 亚基的关键。 Gβ亚基。DAT 功能的改变与多种破坏性神经病理学状况有关;因此,这项工作代表了通过信号蛋白更好地理解 DAT 调节的一步,
更新日期:2020-07-27
中文翻译:

G 蛋白介导的多巴胺转运蛋白调控的结构决定因素。
大脑中的多巴胺清除由多巴胺转运蛋白 (DAT) 控制,DAT 是一种位于质膜中的蛋白质,可驱动细胞外多巴胺再摄取到突触前神经元中。研究表明,异源三聚体 G 蛋白的 βγ 亚基通过与转运蛋白 C 末端区域的物理关联来调节 DAT 功能。Gβγ亚基对神经递质转运蛋白的调节在文献中是前所未有的。因此,研究这种特定蛋白质-蛋白质相互作用的结构细节很有趣。在这里,我们改进了黑腹果蝇DAT (dDAT)的晶体结构,从头建模N-和C-末端结构域;随后,我们使用全长 dDAT 结构生成人类 DAT (hDAT) 的比较模型。两种蛋白质均采用蛋白质-蛋白质对接与 Gβ1γ2 亚基组装,随后进行分子动力学模拟以确定控制 hDAT:Gβγ 和 dDAT:Gβγ 复合物形成的特定相互作用。发现包含 hDAT 中残基 R588 和 dDAT 中 R587 的 [L/F]R[Q/E]R 序列基序是通过与位于顶部的带负电荷残基簇的静电相互作用结合 Gβγ 亚基的关键。 Gβ亚基。DAT 功能的改变与多种破坏性神经病理学状况有关;因此,这项工作代表了通过信号蛋白更好地理解 DAT 调节的一步,



























 京公网安备 11010802027423号
京公网安备 11010802027423号