当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
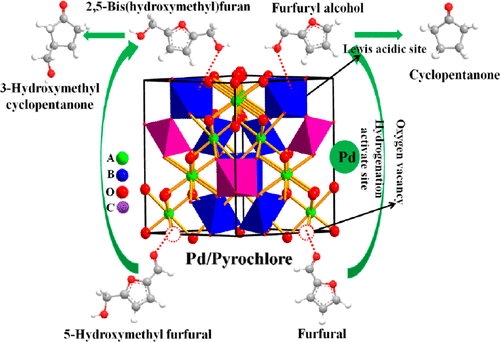
Hydrogenative Ring-Rearrangement of Biobased Furanic Aldehydes to Cyclopentanone Compounds over Pd/Pyrochlore by Introducing Oxygen Vacancies
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-10 , DOI: 10.1021/acscatal.0c01666 Qiang Deng 1 , Rui Gao 1 , Xiang Li 1 , Jun Wang 1 , Zheling Zeng 1 , Ji-Jun Zou 2 , Shuguang Deng 3
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-06-10 , DOI: 10.1021/acscatal.0c01666 Qiang Deng 1 , Rui Gao 1 , Xiang Li 1 , Jun Wang 1 , Zheling Zeng 1 , Ji-Jun Zou 2 , Shuguang Deng 3
Affiliation
|
Upgrading furanic aldehydes (such as furfural or 5-hydroxymethyl furfural) to cyclopentanone compounds (such as cyclopentanone or 3-hydroxymethyl cyclopentanone) is of great significance for the synthesis of high-value chemicals and biomass utilization. Developing an efficient reduced metal/acidic support with Lewis acidity is the key to facilitating the carbonyl hydrogenation and hydrolysis steps in the hydrogenative ring-rearrangement reaction. Herein, three pure Lewis acidic pyrochlore supports of the form A2B2O7 (La2Sn2O7, Y2Sn2O7, and Y2(Sn0.7Ce0.3)2O7-δ) with the same crystal structures and different metals are synthesized. The Lewis acidity and the surface properties of the pyrochlore can be tuned by inserting different kinds of A and B site metals. After impregnation, Pd nanoparticles with appropriate particle sizes are uniformly loaded on the surface of pyrochlore. For the reaction of the furanic aldehydes, all of these pyrochlore-based catalysts exhibit hydrogenation and hydrolysis rates that are both faster than those of traditional support-based catalysts due to the oxygen vacancy and pure Lewis acidity of the support. Among these pyrochlore-based catalysts, Pd/Y2Sn2O7 exhibits activity and selectivity that are higher than those of Pd/La2Sn2O7. Moreover, the Y2Sn2O7-based catalyst partially substituted by Ce3+ ions at the B site is more efficient, with the highest cyclopentanone yield and 3-hydroxymethyl cyclopentanone yield of 95.0% and 92.5%, respectively. Furthermore, the catalyst can still maintain an effective activity and stability after 4 runs. This study not only presents an efficient biobased route for the production of cyclopentanone compounds but also focuses on the acid catalytic performance of pyrochlore based on its pure Lewis acidity.
中文翻译:

通过引入氧空位将生物基呋喃醛在Pd /烧绿石上加氢环重排为环戊酮化合物
将呋喃醛(例如糠醛或5-羟甲基糠醛)升级为环戊酮化合物(例如环戊酮或3-羟甲基环戊酮)对于合成高价值化学品和生物质利用具有重要意义。开发具有路易斯酸性的有效的还原性金属/酸性载体是促进氢化环重排反应中羰基氢化和水解步骤的关键。在此,三个纯的路易斯酸性烧绿石载体为A 2 B 2 O 7(La 2 Sn 2 O 7,Y 2 Sn 2 O 7和Y 2(Sn 0.7 Ce合成具有相同晶体结构和不同金属的0.3)2 O7 -δ)。可以通过插入不同种类的A和B位金属来调节烧绿石的Lewis酸度和表面性质。浸渍后,将具有适当粒径的Pd纳米颗粒均匀地负载在烧绿石的表面上。对于呋喃醛的反应,由于载体的氧空位和纯路易斯酸度,所有这些基于烧绿石的催化剂都显示出比传统的基于载体的催化剂更快的氢化和水解速率。在这些基于烧绿石的催化剂中,Pd / Y 2 Sn 2 O 7具有比Pd / La 2 Sn 2 O 7更高的活性和选择性。此外,在B位点被Ce 3+离子部分取代的基于Y 2 Sn 2 O 7的催化剂更有效,环戊酮的最高收率和3-羟甲基环戊酮的收率分别为95.0%和92.5%。此外,催化剂在4次运行后仍可以保持有效的活性和稳定性。这项研究不仅为生产环戊酮化合物提供了一种有效的基于生物的途径,而且基于其纯的路易斯酸度,着重于烧绿石的酸催化性能。
更新日期:2020-07-02
中文翻译:

通过引入氧空位将生物基呋喃醛在Pd /烧绿石上加氢环重排为环戊酮化合物
将呋喃醛(例如糠醛或5-羟甲基糠醛)升级为环戊酮化合物(例如环戊酮或3-羟甲基环戊酮)对于合成高价值化学品和生物质利用具有重要意义。开发具有路易斯酸性的有效的还原性金属/酸性载体是促进氢化环重排反应中羰基氢化和水解步骤的关键。在此,三个纯的路易斯酸性烧绿石载体为A 2 B 2 O 7(La 2 Sn 2 O 7,Y 2 Sn 2 O 7和Y 2(Sn 0.7 Ce合成具有相同晶体结构和不同金属的0.3)2 O7 -δ)。可以通过插入不同种类的A和B位金属来调节烧绿石的Lewis酸度和表面性质。浸渍后,将具有适当粒径的Pd纳米颗粒均匀地负载在烧绿石的表面上。对于呋喃醛的反应,由于载体的氧空位和纯路易斯酸度,所有这些基于烧绿石的催化剂都显示出比传统的基于载体的催化剂更快的氢化和水解速率。在这些基于烧绿石的催化剂中,Pd / Y 2 Sn 2 O 7具有比Pd / La 2 Sn 2 O 7更高的活性和选择性。此外,在B位点被Ce 3+离子部分取代的基于Y 2 Sn 2 O 7的催化剂更有效,环戊酮的最高收率和3-羟甲基环戊酮的收率分别为95.0%和92.5%。此外,催化剂在4次运行后仍可以保持有效的活性和稳定性。这项研究不仅为生产环戊酮化合物提供了一种有效的基于生物的途径,而且基于其纯的路易斯酸度,着重于烧绿石的酸催化性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号