当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of cis and trans 4‐Aminopipecolic Acids as γ‐Amino Acids for the Construction of Cyclic RGD‐Containing Peptidomimetics Antagonists of αVβ3 Integrin
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1002/ejoc.202000634 Francesca Dordoni 1 , Dina Scarpi 1 , Francesca Bianchini 2 , Alessandro Contini 3 , Ernesto G. Occhiato 1
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-06-11 , DOI: 10.1002/ejoc.202000634 Francesca Dordoni 1 , Dina Scarpi 1 , Francesca Bianchini 2 , Alessandro Contini 3 , Ernesto G. Occhiato 1
Affiliation
|
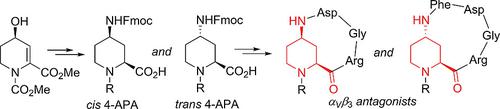
A stereodivergent preparation of cis and trans 4‐aminopipecolic acids (4‐APAs) was developed from a common precursor to obtain suitably protected, constrained γ‐amino acids useful in peptidomimetic synthesis. Two antagonists of αVβ3 integrin were synthesized.

中文翻译:

对映体合成顺式和反式4-氨基哌酸作为γ-氨基酸,用于构建含环RGD的拟肽类αVβ3整联蛋白拮抗剂。
从常见的前体中开发出了顺式和反式4-氨基哌酸(4-APA)的立体发散剂,以获得可适当保护,受约束的γ-氨基酸,可用于拟肽合成。α的两个拮抗剂V β 3整联合成。

更新日期:2020-07-24

中文翻译:

对映体合成顺式和反式4-氨基哌酸作为γ-氨基酸,用于构建含环RGD的拟肽类αVβ3整联蛋白拮抗剂。
从常见的前体中开发出了顺式和反式4-氨基哌酸(4-APA)的立体发散剂,以获得可适当保护,受约束的γ-氨基酸,可用于拟肽合成。α的两个拮抗剂V β 3整联合成。




























 京公网安备 11010802027423号
京公网安备 11010802027423号