当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
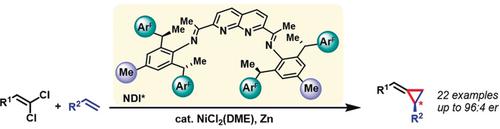
A Chiral Naphthyridine Diimine Ligand Enables Nickel-Catalyzed Asymmetric Alkylidenecyclopropanations.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-06-10 , DOI: 10.1002/anie.202006082 Elena Braconi 1 , Nicolai Cramer 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-06-10 , DOI: 10.1002/anie.202006082 Elena Braconi 1 , Nicolai Cramer 1
Affiliation
|
A novel class of chiral naphthyridine diimine ligands (NDI*) readily accessible from C2‐symmetric 2,6‐di‐(1‐arylethyl)anilines is described. The utility of these ligands, particularly one with fluorinated aryl side arms, is demonstrated by a reductive Ni‐catalyzed enantioselective alkylidene transfer reaction from 1,1‐dichloroalkenes to olefins. This transformation provides direct access to a broad range of synthetically valuable alkylidenecyclopropanes in high yields and enantioselectivities.
中文翻译:

手性萘啶二亚胺配体使镍催化不对称亚烷基环丙烷化。
描述了一种新型的手性萘啶二亚胺配体(NDI *),可从C 2对称的2,6-二-(1-芳基乙基)苯胺轻松获得。这些配体,特别是带有氟化芳基侧臂的配体的实用性,是通过还原性镍催化的对映选择性亚烷基从1,1-二氯烯烃到烯烃的转移反应来证明的。这种转化可以以高收率和对映选择性直接获得各种合成有价值的亚烷基环丙烷。
更新日期:2020-06-10
中文翻译:

手性萘啶二亚胺配体使镍催化不对称亚烷基环丙烷化。
描述了一种新型的手性萘啶二亚胺配体(NDI *),可从C 2对称的2,6-二-(1-芳基乙基)苯胺轻松获得。这些配体,特别是带有氟化芳基侧臂的配体的实用性,是通过还原性镍催化的对映选择性亚烷基从1,1-二氯烯烃到烯烃的转移反应来证明的。这种转化可以以高收率和对映选择性直接获得各种合成有价值的亚烷基环丙烷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号