Molecular Therapy - Methods & Clinical Development ( IF 4.7 ) Pub Date : 2020-06-11 , DOI: 10.1016/j.omtm.2020.05.031 Victoria Gálvez 1, 2, 3 , Esteban Chacón-Solano 2, 3, 4 , Jose Bonafont 2, 3, 4 , Ángeles Mencía 1, 2, 3 , Wei-Li Di 5 , Rodolfo Murillas 1, 2, 3 , Sara Llames 2, 3, 6 , Asunción Vicente 7, 8 , Marcela Del Rio 2, 3, 4 , Marta Carretero 1, 2, 3 , Fernando Larcher 1, 2, 3, 4
|
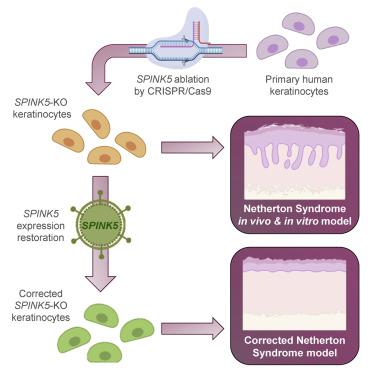
Current efforts to find specific genodermatoses treatments and define precise pathogenesis mechanisms require appropriate surrogate models with human cells. Although transgenic and gene knockout mouse models for several of these disorders exist, they often fail to faithfully replicate the clinical and histopathological features of the human skin condition. We have established a highly efficient method for precise deletion of critical gene sequences in primary human keratinocytes, based on CRISPR-Cas9-mediated gene editing. Using this methodology, in the present study we generated a model of Netherton syndrome by disruption of SPINK5. Gene-edited cells showed absence of LEKTI expression and were able to recapitulate a hyperkeratotic phenotype with most of the molecular hallmarks of Netherton syndrome, after grafting to immunodeficient mice and in organotypic cultures. To validate the model as a platform for therapeutic intervention, we tested an ex vivo gene therapy approach using a lentiviral vector expressing SPINK5. Re-expression of SPINK5 in an immortalized clone of SPINK5-knockout keratinocytes was capable of reverting from Netherton syndrome to a normal skin phenotype in vivo and in vitro. Our results demonstrate the feasibility of modeling genodermatoses, such as Netherton syndrome, by efficiently disrupting the causative gene to better understand its pathogenesis and to develop novel therapeutic approaches.
中文翻译:

人类角质形成细胞中高效CRISPR-Cas9介导的基因消融以概括基因皮肤病:Netherton综合征的建模。
当前寻找特定的基因皮肤病治疗方法和定义精确的发病机理的努力需要适当的人类细胞替代模型。尽管存在针对这些疾病中的几种的转基因和基因敲除小鼠模型,但它们通常无法忠实地复制人类皮肤状况的临床和组织病理学特征。基于CRISPR-Cas9介导的基因编辑,我们已经建立了一种高效的方法来精确缺失人类原代角质形成细胞中的关键基因序列。使用这种方法,在本研究中,我们通过破坏SPINK5生成了Netherton综合征模型。经过基因编辑的细胞移植到免疫缺陷小鼠和器官型培养物中后,显示出不存在LEKTI表达,并且能够以Netherton综合征的大多数分子特征重现过度角化的表型。为了验证该模型是否可作为治疗干预的平台,我们使用表达SPINK5的慢病毒载体测试了离体基因治疗方法。的重新表达SPINK5中的永生化克隆SPINK5敲除的角质形成细胞是能够从瑟顿综合征回复到正常皮肤的表型的体内和体外。我们的结果表明,通过有效破坏病因基因以更好地了解其发病机理并开发新的治疗方法,可以对诸如奈瑟顿综合症等基因皮肤病进行建模。



























 京公网安备 11010802027423号
京公网安备 11010802027423号