Joule ( IF 39.8 ) Pub Date : 2020-06-11 , DOI: 10.1016/j.joule.2020.05.004 William E. Gent , Iwnetim Iwnetu Abate , Wanli Yang , Linda F. Nazar , William C. Chueh
|
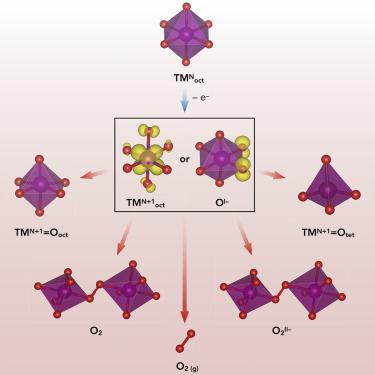
High-valent redox, where over-oxidation of oxygen or transition metals (TMs) drives extensive charge sharing through the formation of short covalent bonds, has historically been avoided in intercalation electrodes because of its association with structural disorder and electrochemical irreversibility. Here, we present a perspective on the origin of these undesirable behaviors and materials design criteria to mitigate them. Drawing parallels between oxygen redox and high-valent TM redox (e.g., CrIII/VI and VIII/V), we reveal that the defect formation energy landscape is the primary factor controlling the electrochemical reversibility of high-valent redox, as it determines which defects form to accommodate the short covalent bonds as well as the nature of those covalent bonding arrangements. By tuning the defect formation energy landscape, researchers can control the nature of the oxidized species while minimizing structural disorder. These concepts reveal a wide range of previously avoided redox mechanisms as promising candidates for high density energy storage.
中文翻译:

嵌入电极中高价氧化还原的设计规则
从历史上讲,在高价氧化还原中,氧或过渡金属(TMs)的过氧化会通过形成短的共价键来驱动广泛的电荷共享,而在高价氧化还原中,由于其与结构无序和电化学不可逆性相关,因此已被避免。在这里,我们对这些不良行为的根源和减轻它们的材料设计标准提出了看法。在氧气氧化还原和高价TM氧化还原(例如Cr III / VI和V III / V),我们发现缺陷形成的能量格局是控制高价氧化还原的电化学可逆性的主要因素,因为它决定了哪种缺陷形式可以容纳短的共价键以及那些共价键排列的性质。通过调整缺陷形成的能量分布,研究人员可以控制氧化物种的性质,同时最大程度地减少结构紊乱。这些概念揭示了广泛使用的先前避免的氧化还原机制,作为高密度储能的有希望的候选者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号