Environmental Research ( IF 8.3 ) Pub Date : 2020-06-11 , DOI: 10.1016/j.envres.2020.109767 Van Luan Pham 1 , Do-Gun Kim 1 , Seok-Oh Ko 1
|
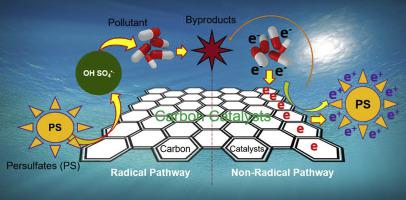
Two representative carbon catalysts, granular activated carbon (GAC) and carbon nanotube (CNT) were selected for the degradation of acetaminophen (ACT) by persulfate (PS) activation. In this study, the materials characterization study indicated that the surface functional groups and surface area of the GAC were more abundant and larger, respectively, than the CNT. Whereas the contribution of sp2 and CO groups of the CNT was superior to the GAC; the structural defect levels and surface charging characteristics (pHPZC) were similar in both. The mass-based pseudo-first-order reaction rate constant of the CNT was 1.5 m−1 g−1, which was 50 times higher than that of the GAC (0.03 m−1 g−1). Radical and non-radical pathways were evaluated for catalytic reactions by the GAC/PS and CNT/PS systems, respectively. Experimental results using scavenger tests, linear voltammetry, and electron spin resonance (ESR) showed that the radical pathway was dominant in the GAC/PS system, whereas the non-radical pathway was dominant in the CNT/PS systems. To confirm this, the influence of affecting factors such as initial ACT concentration, the dosage of oxidant (PS), and initial pH were also investigated and compared for the two systems. The results showed that the catalytic activity of the GAC/PS system was highly dependent on initial concentrations of ACT and PS, while these were less influential in the CNT/PS system. The removal efficiency of ACT was not affected under a pH of 3–7 in both systems. Reusability experiments were conducted five times, and both the CNT/PS and GAC/PS systems demonstrated that the removal rate of ACT did not notably decrease with the number of experiment repetitions. This means that the application of a carbon catalyst to treat pharmaceutical contaminants in wastewater is effective.
中文翻译:

碳催化剂对乙酰氨基酚的高级氧化降解:自由基和非自由基途径。
选择了两种代表性的碳催化剂,颗粒状活性炭(GAC)和碳纳米管(CNT),用于通过过硫酸盐(PS)活化降解对乙酰氨基酚(ACT)。在这项研究中,材料表征研究表明,GAC的表面官能团和表面积分别比CNT更丰富和更大。CNT的sp 2和C O基团的贡献优于GAC;两者的结构缺陷水平和表面电荷特性(pH PZC)相似。CNT的基于质量的拟一级反应速率常数为1.5 m -1 g -1,比GAC(0.03 m -1 g -1)高50倍。)。分别通过GAC / PS和CNT / PS系统评估了自由基和非自由基途径的催化反应。使用清除剂测试,线性伏安法和电子自旋共振(ESR)进行的实验结果表明,自由基途径在GAC / PS系统中占主导地位,而非自由基途径在CNT / PS系统中占主导地位。为了证实这一点,还研究并比较了两个系统的影响因素,如初始ACT浓度,氧化剂剂量(PS)和初始pH的影响。结果表明,GAC / PS系统的催化活性高度依赖于ACT和PS的初始浓度,而对CNT / PS系统的影响较小。在两个系统中,pH值为3-7时,ACT的去除效率均不受影响。可重复使用性实验进行了五次,并且CNT / PS和GAC / PS系统均表明ACT的去除率不会随着实验重复次数的增加而显着下降。这意味着将碳催化剂用于处理废水中的药物污染物是有效的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号