当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
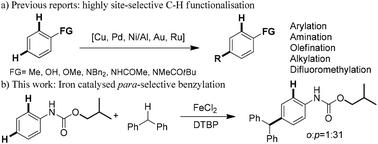
The protecting group enabled para-selective C–H benzylation of anilides via iron(II) catalysis: a convenient approach for the synthesis of triarylmethanes
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0qo00402b Yi Han 1, 2, 3, 4, 5 , Guobao Li 1, 2, 3, 4, 5 , Lingling Liu 1, 2, 3, 4, 5 , Chenyang Dai 1, 2, 3, 4, 5 , Da-Qing Shi 1, 2, 3, 4, 5 , Yingsheng Zhao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0qo00402b Yi Han 1, 2, 3, 4, 5 , Guobao Li 1, 2, 3, 4, 5 , Lingling Liu 1, 2, 3, 4, 5 , Chenyang Dai 1, 2, 3, 4, 5 , Da-Qing Shi 1, 2, 3, 4, 5 , Yingsheng Zhao 1, 2, 3, 4, 5
Affiliation
|
We report the para-selective C–H benzylation of phenyl carbamate with an iron(II) catalyst. This new method provides an efficient and convenient approach for the synthesis of triarylmethanes starting from readily available anilines and diphenylmethane. Preliminary mechanistic studies revealed that the iron(II) catalyst was essential and the use of the sterically demanding isobutoxy group led to high para-selectivity.
中文翻译:

该保护基能够通过铁(II)催化实现对苯甲酸酯的对-选择性苄基甲烷苄基化:一种方便的合成三芳基甲烷的方法
我们报道了氨基苯甲酸与铁(II)催化剂的对位选择性C–H苄基化。这种新方法为从容易获得的苯胺和二苯甲烷开始合成三芳基甲烷提供了一种高效便捷的方法。初步的机理研究表明,铁(II)催化剂是必不可少的,并且使用空间要求高的异丁氧基可导致较高的对位选择性。
更新日期:2020-07-14
中文翻译:

该保护基能够通过铁(II)催化实现对苯甲酸酯的对-选择性苄基甲烷苄基化:一种方便的合成三芳基甲烷的方法
我们报道了氨基苯甲酸与铁(II)催化剂的对位选择性C–H苄基化。这种新方法为从容易获得的苯胺和二苯甲烷开始合成三芳基甲烷提供了一种高效便捷的方法。初步的机理研究表明,铁(II)催化剂是必不可少的,并且使用空间要求高的异丁氧基可导致较高的对位选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号