当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sharp-tip enhanced catalytic CO oxidation by atomically dispersed Pt1/Pt2 on a raised graphene oxide platform
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0ta04156d Chuanyi Jia 1, 2, 3, 4, 5 , Yujin Zhang 5, 6, 7, 8, 9 , Xijun Wang 10, 11, 12, 13, 14 , Wenhui Zhong 1, 2, 3, 4, 5 , Oleg V. Prezhdo 15, 16, 17, 18 , Yi Luo 10, 11, 12, 13, 14 , Jun Jiang 10, 11, 12, 13, 14
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-06-10 , DOI: 10.1039/d0ta04156d Chuanyi Jia 1, 2, 3, 4, 5 , Yujin Zhang 5, 6, 7, 8, 9 , Xijun Wang 10, 11, 12, 13, 14 , Wenhui Zhong 1, 2, 3, 4, 5 , Oleg V. Prezhdo 15, 16, 17, 18 , Yi Luo 10, 11, 12, 13, 14 , Jun Jiang 10, 11, 12, 13, 14
Affiliation
|
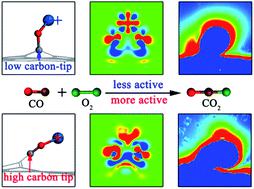
Revealing structure–activity relationships of graphene-supported atomically dispersed transition metal catalysts is of key importance in catalysis chemistry and materials science. Here we report a first-principles theoretical study on O2 activation and CO oxidation catalyzed by atomically dispersed Pt1/Pt2 anchored on a raised graphene (Gr) platform. The unique sharp-tip structure of the protuberant graphene platform (carbon-tip) can collect extra polarization electrons on the Pt-tip, promoting electron transfer between the catalyst and adsorbates, and greatly enhancing the chemical activity. Higher carbon-tips on defective graphene oxide with a carbon vacancy (Gr-V) produce more polarization electrons, a more localized electric field, and a larger up-shift of the d-orbital center of the Pt-tip, resulting in better catalytic performance. The carbon-tip enhancement reduces the energy barrier for the CO oxidation on Pt1O2/Gr-V from 1.19 eV to 0.56 eV compared with Pt1 on planar Gr. In addition, CO poisoning is significantly alleviated, with the CO poisoning rate Γθ reduced from 2.00 eV to 0.52 eV. Moreover, a linear relationship between the activation barrier and the binding energy of adsorbates is found for various atomically dispersed Pt-tip catalysts on the raised graphene oxide platform. Importantly, the order of catalytic activity is consistent with the carbon-tip enhancement, i.e., Pt1O2/Gr-V > Pt2O4/Gr-V > Pt1O2/Gr > Pt2O4/Gr. These findings provide important guidance for rational design of atomically dispersed catalysts.
中文翻译:

通过在升高的氧化石墨烯平台上原子分散的Pt1 / Pt2来增强尖端的催化CO氧化
揭示石墨烯负载的原子分散过渡金属催化剂的结构-活性关系在催化化学和材料科学中至关重要。在这里,我们报告有关原子分散的Pt 1 / Pt 2催化O 2活化和CO氧化的第一原理理论研究固定在凸起的石墨烯(Gr)平台上。突出的石墨烯平台的独特尖端结构(碳尖端)可以在Pt尖端上收集额外的极化电子,从而促进催化剂与被吸附物之间的电子转移,并大大增强化学活性。有缺陷的氧化石墨烯上的碳尖端具有较高的碳空位(Gr-V),可产生更多的极化电子,更局限的电场以及Pt尖端的d轨道中心更大的上移,从而产生更好的催化作用性能。与Pt 1相比,碳尖端增强功能将Pt 1 O 2 / Gr-V上的CO氧化的能垒从1.19 eV降低到0.56 eV在平面Gr上 此外,CO中毒显著缓解,与CO中毒率Γ θ从2.00电子伏特降低到0.52电子伏特。此外,对于在升高的氧化石墨烯平台上的各种原子分散的Pt-tip催化剂,发现了活化势垒与被吸附物的结合能之间的线性关系。重要的是,催化活性的顺序与碳尖端增强一致,即Pt 1 O 2 / Gr-V> Pt 2 O 4 / Gr-V> Pt 1 O 2 / Gr> Pt 2 O 4 / Gr。这些发现为合理设计原子分散催化剂提供了重要指导。
更新日期:2020-06-30
中文翻译:

通过在升高的氧化石墨烯平台上原子分散的Pt1 / Pt2来增强尖端的催化CO氧化
揭示石墨烯负载的原子分散过渡金属催化剂的结构-活性关系在催化化学和材料科学中至关重要。在这里,我们报告有关原子分散的Pt 1 / Pt 2催化O 2活化和CO氧化的第一原理理论研究固定在凸起的石墨烯(Gr)平台上。突出的石墨烯平台的独特尖端结构(碳尖端)可以在Pt尖端上收集额外的极化电子,从而促进催化剂与被吸附物之间的电子转移,并大大增强化学活性。有缺陷的氧化石墨烯上的碳尖端具有较高的碳空位(Gr-V),可产生更多的极化电子,更局限的电场以及Pt尖端的d轨道中心更大的上移,从而产生更好的催化作用性能。与Pt 1相比,碳尖端增强功能将Pt 1 O 2 / Gr-V上的CO氧化的能垒从1.19 eV降低到0.56 eV在平面Gr上 此外,CO中毒显著缓解,与CO中毒率Γ θ从2.00电子伏特降低到0.52电子伏特。此外,对于在升高的氧化石墨烯平台上的各种原子分散的Pt-tip催化剂,发现了活化势垒与被吸附物的结合能之间的线性关系。重要的是,催化活性的顺序与碳尖端增强一致,即Pt 1 O 2 / Gr-V> Pt 2 O 4 / Gr-V> Pt 1 O 2 / Gr> Pt 2 O 4 / Gr。这些发现为合理设计原子分散催化剂提供了重要指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号