当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thioesterase from Cereulide Biosynthesis Is Responsible for Oligomerization and Macrocyclization of a Linear Tetradepsipeptide.
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jnatprod.0c00333 Graham W Heberlig 1 , Christopher N Boddy 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jnatprod.0c00333 Graham W Heberlig 1 , Christopher N Boddy 1
Affiliation
|
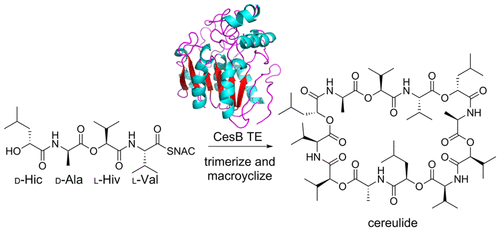
Cereulide is a toxic cyclic depsidodecapeptide produced in Bacillus cereus by two nonribosomal peptide synthetases, CesA and CesB. While highly similar in structure to valinomycin and with a homologous biosynthetic gene cluster, recent work suggests that cereulide is produced via a different mechanism that relies on a noncanonical coupling of two didepsipeptide-peptidyl carrier protein (PCP) bound intermediates. Ultimately this alternative mechanism generates a tetradepsipeptide-PCP bound intermediate that differs from the tetradepsipeptide-PCP intermediate predicted from canonical activity of CesA and CesB. To differentiate between the mechanisms, both tetradepsipeptides were prepared as N-acetyl cysteamine thioesters (SNAC), and the ability of the purified recombinant terminal CesB thioesterase (CesB TE) to oligomerize and macrocyclize each substrate was probed. Only the canonical substrate is converted to cereulide, ruling out the alternative mechanism. It was demonstrated that CesB TE can use related tetradepsipeptide substrates, such as the valinomycin tetradespipetide and a hybrid cereulide–valinomycin tetradepsipetide in conjunction with its native substrate to generate chimeric natural products. This work clarifies the biosynthetic origins of cereulide and provides a powerful biocatalyst to access analogues of these ionophoric natural products.
中文翻译:

来自脑脑苷脂生物合成的硫酯酶负责线性四肽的寡聚化和大环化。
Cereulide 是一种有毒的环状 depsidodecapeptide,由两种非核糖体肽合成酶 CesA 和 CesB在蜡样芽孢杆菌中产生。虽然在结构上与缬氨霉素高度相似,并具有同源的生物合成基因簇,但最近的工作表明,脑蓝素是通过一种不同的机制产生的,该机制依赖于两个二缩肽-肽基载体蛋白 (PCP) 结合中间体的非规范偶联。最终,这种替代机制产生了一种四肽-PCP 结合中间体,它不同于根据 CesA 和 CesB 的规范活性预测的四肽-PCP 中间体。为了区分机制,将两种四肽制备为N-乙酰半胱胺硫酯 (SNAC),以及纯化的重组末端 CesB 硫酯酶 (CesB TE) 寡聚化和大环化每种底物的能力。只有经典底物被转化为脑脑苷脂,排除了替代机制。结果表明,CesB TE 可以使用相关的四肽底物,如缬氨霉素四肽和混合脑苷脂-缬氨霉素四肽与其天然底物结合产生嵌合天然产物。这项工作阐明了脑苷脂的生物合成起源,并提供了一种强大的生物催化剂来获取这些离子载体天然产物的类似物。
更新日期:2020-06-26
中文翻译:

来自脑脑苷脂生物合成的硫酯酶负责线性四肽的寡聚化和大环化。
Cereulide 是一种有毒的环状 depsidodecapeptide,由两种非核糖体肽合成酶 CesA 和 CesB在蜡样芽孢杆菌中产生。虽然在结构上与缬氨霉素高度相似,并具有同源的生物合成基因簇,但最近的工作表明,脑蓝素是通过一种不同的机制产生的,该机制依赖于两个二缩肽-肽基载体蛋白 (PCP) 结合中间体的非规范偶联。最终,这种替代机制产生了一种四肽-PCP 结合中间体,它不同于根据 CesA 和 CesB 的规范活性预测的四肽-PCP 中间体。为了区分机制,将两种四肽制备为N-乙酰半胱胺硫酯 (SNAC),以及纯化的重组末端 CesB 硫酯酶 (CesB TE) 寡聚化和大环化每种底物的能力。只有经典底物被转化为脑脑苷脂,排除了替代机制。结果表明,CesB TE 可以使用相关的四肽底物,如缬氨霉素四肽和混合脑苷脂-缬氨霉素四肽与其天然底物结合产生嵌合天然产物。这项工作阐明了脑苷脂的生物合成起源,并提供了一种强大的生物催化剂来获取这些离子载体天然产物的类似物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号