当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Use of neomycin as a structured amino-containing side chain motif for phenanthroline-based G-quadruplex ligands and telomerase inhibitors.
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-06-09 , DOI: 10.1111/cbdd.13741 Mandeep Singh 1 , Siwen Wang 1 , Hyun Joo 1 , Zhihan Ye 1 , Krege M Christison 1 , Ryan Hekman 2 , Craig Vierra 2 , Liang Xue 1
Chemical Biology & Drug Design ( IF 3 ) Pub Date : 2020-06-09 , DOI: 10.1111/cbdd.13741 Mandeep Singh 1 , Siwen Wang 1 , Hyun Joo 1 , Zhihan Ye 1 , Krege M Christison 1 , Ryan Hekman 2 , Craig Vierra 2 , Liang Xue 1
Affiliation
|
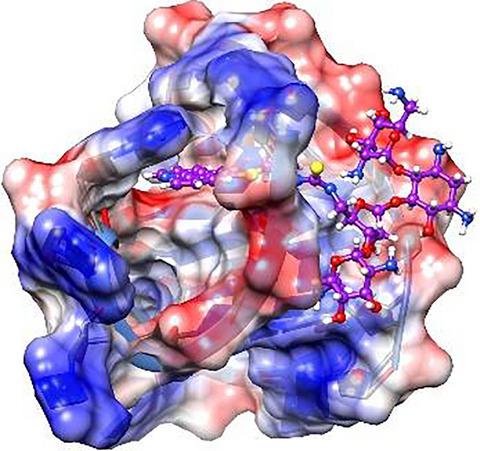
In this paper, we report the synthesis of a phenanthroline and neomycin conjugate (7). Compound 7 binds to a human telomeric G‐quadruplex (G1) with a higher affinity compared with its parent compounds (phenanthroline and neomycin), which is determined by several biophysical studies. Compound 7 shows good selectivity for G‐quadruplex (G4) DNA over duplex DNA. The binding of 7 with G1 is predominantly enthalpy‐driven, and the binding stoichiometry of 7 with G1 is one for the tight‐binding event as determined by ESI mass spectrometry. A plausible binding mode is a synergistic effect of end‐stacking and groove interactions, as indicated by docking studies. Compound 7 can inhibit human telomerase activity at low micromolar concentrations, which is more potent than previously reported 5‐substituted phenanthroline derivatives.
中文翻译:

使用新霉素作为结构化的含氨基侧链基序,用于基于菲咯啉的 G-四链体配体和端粒酶抑制剂。
在本文中,我们报告了菲咯啉和新霉素偶联物的合成 ( 7 )。化合物7与其母体化合物(菲咯啉和新霉素)相比,以更高的亲和力与人端粒 G-四链体 ( G1 ) 结合,这是由多项生物物理学研究确定的。与双链 DNA 相比,化合物7对 G-四链体 (G4) DNA 显示出良好的选择性。7与G1的结合主要受焓驱动,7与G1的结合化学计量是由 ESI 质谱法确定的紧束缚事件之一。对接研究表明,一种合理的结合模式是末端堆叠和凹槽相互作用的协同效应。化合物7可以在低微摩尔浓度下抑制人端粒酶活性,这比先前报道的 5-取代菲咯啉衍生物更有效。
更新日期:2020-06-09
中文翻译:

使用新霉素作为结构化的含氨基侧链基序,用于基于菲咯啉的 G-四链体配体和端粒酶抑制剂。
在本文中,我们报告了菲咯啉和新霉素偶联物的合成 ( 7 )。化合物7与其母体化合物(菲咯啉和新霉素)相比,以更高的亲和力与人端粒 G-四链体 ( G1 ) 结合,这是由多项生物物理学研究确定的。与双链 DNA 相比,化合物7对 G-四链体 (G4) DNA 显示出良好的选择性。7与G1的结合主要受焓驱动,7与G1的结合化学计量是由 ESI 质谱法确定的紧束缚事件之一。对接研究表明,一种合理的结合模式是末端堆叠和凹槽相互作用的协同效应。化合物7可以在低微摩尔浓度下抑制人端粒酶活性,这比先前报道的 5-取代菲咯啉衍生物更有效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号