Tetrahedron ( IF 2.1 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.tet.2020.131338 Soumitra Guin , Hemonta K. Saha , Ashvani K. Patel , Santosh K. Gudimella , Subhankar Biswas , Sampak Samanta
|
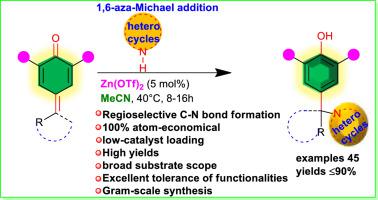
An efficient Zn(OTf)2-catalyzed regioselective C–N bond making reaction between a bunch of aryl/heteroaryl-substituted para-quinone methides as ideal 1,6-acceptors and various aromatic/non-aromatic aza-heterocycles bearing N–H moiety namely carbazoles, pyrazoles, indazole, benzotriazole and saccharin is reported. This 1,6-aza-Michael technique delivers predominantly N1-diarylmethyl-substituted heterocyclic scaffolds bearing a valuable phenolic moiety in good to high yields with an excellent regioselectivity. Furthermore, this LUMO lowering catalytic system allows different kinds of useful functionalities and excels with broad substrates under mild conditions. Importantly, our control experiments suggested that N2-adducts of indazole, benzotriazole and 3-methyl pyrazole as minor isomers were progressively converted into N1-adducts during the reaction via a retro-aza-Michael reaction triggered by Zn(OTf)2, offering excellent regioselectivities of the products.
中文翻译:

Zn(OTf)2催化的带有N-杂环的1,6-Aza-Michael加成对-醌甲基化物:N-二芳基甲基取代的杂环的区域选择性方法
一堆芳基/杂芳基取代的对位之间有效的Zn(OTf)2催化的区域选择性C–N键反应据报道,甲基苯醌是理想的1,6-受体和各种带有N–H部分的芳香族/非芳香族氮杂杂环,即咔唑,吡唑,吲唑,苯并三唑和糖精。这种1,6-氮杂-迈克尔技术主要是通过N1-二芳基甲基取代的杂环骨架以高产率到高产率提供具有有价值的酚部分的区域,并具有出色的区域选择性。此外,这种LUMO降低催化体系可实现各种有用的功能,并且在温和条件下可与宽范围的底物一起使用。重要的是,我们的对照实验表明,吲哚,苯并三唑和3-甲基吡唑的N2加成物是次要异构体,在反应过程中通过Zn(OTf)2引发的逆向aza-Michael反应逐渐转化为N1加成物。,提供极好的区域选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号