Protein Expression and Purification ( IF 1.6 ) Pub Date : 2020-06-10 , DOI: 10.1016/j.pep.2020.105679 Jie Zhu 1 , Kun Yang 1 , Aijie Liu 2 , Xiaoxue Lu 1 , Linsong Yang 1 , Qinghuan Zhao 3
|
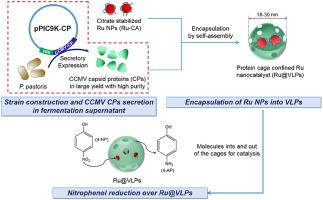
The applications of viral protein cages have expanded rapidly into the fields of bionanotechnology and materials science. However, the low-cost production of viral capsid proteins (CPs) on a large scale is always a challenge. Herein, we develop a highly efficient expression system by constructing recombinant Pichia pastoris cells as a “factory” for the secretion of soluble cowpea chlorotic mottle virus (CCMV) CPs. Under optimal induction conditions (0.9 mg/mL of methanol concentration at 30 °C for 96 h), a high yield of approximately 95 mg/L of CCMV CPs was harvested from the fermentation supernatant with CPs purity >90%, which has significantly simplified the rest of the purification process. The resultant CPs are employed to encapsulate Ruthenium (Ru) nanoparticles (NPs) via in-vitro self-assembly to prepare hybrid nanocatalyst, i.e. Ru@virus-like particles (VLPs). The catalytic activity over Ru@VLPs was evaluated by reducing 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). The results indicate that, with the protection of protein cages, Ru NPs were highly stabilized during the catalytic reaction. This results in enhanced catalytic activity (reaction rate constant k = 0.14 min−1) in comparison with unsupported citrate-stabilized Ru NPs (Ru-CA) (k = 0.08 min−1). Additionally, comparatively lower activation energy over Ru@VLPs (approximately 32 kJ/mol) than that over Ru-CA (approximately 39 kJ/mol) could be attributed to the synergistic effect between Ru NPs and some functional groups such as amino groups (–NH2) on CPs that weakened the activation barrier of 4-NP reduction. Therefore, enhanced activity and decreased activation energy over Ru@VLPs demonstrated the superiority of Ru@VLPs to unsupported Ru-CA.
中文翻译:

重组cow豆褪绿斑驳病毒衣壳蛋白在巴斯德毕赤酵母中的高分泌表达和钌纳米颗粒的体外封装用于催化。
病毒蛋白笼的应用已迅速扩展到生物纳米技术和材料科学领域。然而,大规模生产低成本的衣壳蛋白一直是一个挑战。在这里,我们通过构建重组巴斯德毕赤酵母来开发高效表达系统细胞是分泌可溶性cow豆绿叶斑驳病毒(CCMV)CP的“工厂”。在最佳诱导条件下(在30°C下96h浓度为0.9 mg / mL甲醇),从CP纯度> 90%的发酵上清液中收获了约95 mg / L的CCMV CP高产量,这大大简化了其余的纯化过程。所得CP被用于通过体外自组装包封钌(Ru)纳米颗粒(NP)以制备杂化纳米催化剂,即Ru @病毒样颗粒(VLP)。通过将4-硝基苯酚(4-NP)还原为4-氨基苯酚(4-AP)来评估Ru @ VLP的催化活性。结果表明,在蛋白质笼的保护下,Ru NPs在催化反应过程中高度稳定。这导致增强的催化活性(反应速率常数与未支持的柠檬酸盐稳定的Ru NPs(Ru-CA)相比,k = 0.14 min -1)(k = 0.08 min -1)。此外,Ru @ VLPs的活化能(约32 kJ / mol)比Ru @ CA的活化能(约39 kJ / mol)要低,这归因于Ru NPs与某些官能团(例如氨基)之间的协同作用。CP上的NH 2)减弱了4-NP还原的激活屏障。因此,与Ru @ VLP相比,增强的活性和降低的活化能证明Ru @ VLP优于不支持的Ru-CA。



























 京公网安备 11010802027423号
京公网安备 11010802027423号