当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparison of disaccharide donors for heparan sulfate synthesis: uronic acids vs. their pyranose equivalents.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-09 , DOI: 10.1039/d0ob00671h Daniel J Sheppard 1 , Scott A Cameron 1 , Peter C Tyler 1 , Ralf Schwörer 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-06-09 , DOI: 10.1039/d0ob00671h Daniel J Sheppard 1 , Scott A Cameron 1 , Peter C Tyler 1 , Ralf Schwörer 1
Affiliation
|
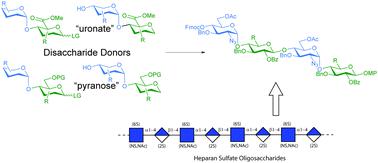
Late oxidation of hexose based building blocks or the use of uronic acid containing building blocks are two complementary strategies in the synthesis of glycosaminoglycans, the latter simplifiying the later stages of the process. Here we report the synthesis and evaluation of various disaccharide donors—uronic acids and their pyranose equivalents—for the synthesis of heparan sulfate, using an established protective group strategy. Hexose based “imidate” type donors perform well in the studied glycosylations, while their corresponding uronate esters fall short; a uronate ester thioglycoside performs equal to, if not better than, a hexose thioglycoside equivalent.
中文翻译:

硫酸乙酰肝素合成的二糖供体的比较:糖醛酸与其吡喃糖当量。
己糖基结构单元的后期氧化或含糖醛酸的结构单元的使用是糖胺聚糖合成中的两种互补策略,后者简化了该过程的后期阶段。在这里,我们使用既定的保护基团策略,报告了各种二糖供体(糖醛酸及其吡喃糖等效物)的合成和评估,用于硫酸乙酰肝素的合成。以己糖为基础的“亚氨酸酯”型供体在所研究的糖基化反应中表现良好,而其相应的尿酸酯则不足。尿酸酯硫糖苷的性能与己糖硫糖苷相当,甚至更好。
更新日期:2020-07-01
中文翻译:

硫酸乙酰肝素合成的二糖供体的比较:糖醛酸与其吡喃糖当量。
己糖基结构单元的后期氧化或含糖醛酸的结构单元的使用是糖胺聚糖合成中的两种互补策略,后者简化了该过程的后期阶段。在这里,我们使用既定的保护基团策略,报告了各种二糖供体(糖醛酸及其吡喃糖等效物)的合成和评估,用于硫酸乙酰肝素的合成。以己糖为基础的“亚氨酸酯”型供体在所研究的糖基化反应中表现良好,而其相应的尿酸酯则不足。尿酸酯硫糖苷的性能与己糖硫糖苷相当,甚至更好。


























 京公网安备 11010802027423号
京公网安备 11010802027423号