当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Steam reforming of formaldehyde for generating hydrogen and coproducing carbon nanotubes for enhanced photosynthesis
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-06-09 , DOI: 10.1039/d0cy00843e Qijie Jin 1, 2, 3, 4, 5 , Aodi Wang 5, 6, 7, 8, 9 , Bingxu Lu 1, 2, 3, 4, 5 , Xin Xu 1, 2, 3, 4, 5 , Yuesong Shen 1, 2, 3, 4, 5 , Yanwei Zeng 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2020-06-09 , DOI: 10.1039/d0cy00843e Qijie Jin 1, 2, 3, 4, 5 , Aodi Wang 5, 6, 7, 8, 9 , Bingxu Lu 1, 2, 3, 4, 5 , Xin Xu 1, 2, 3, 4, 5 , Yuesong Shen 1, 2, 3, 4, 5 , Yanwei Zeng 1, 2, 3, 4, 5
Affiliation
|
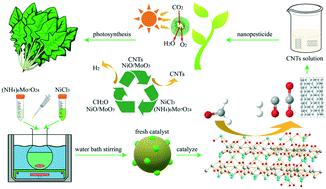
Nickel based catalysts exhibit high catalytic activity, but carbon deposition (coke poisoning) occurs in the field of steam reforming. To solve the coke poisoning of nickel based catalysts, a NiO/MoO3 catalyst was used for the formaldehyde steam reforming reaction, and coproduced carbon nanotubes (CNTs) were exploratively applied to improve plant photosynthesis. Results showed that the NiO/MoO3 catalyst exhibited the highest H2 selectivity of 104.2% at 600 °C, and its initiation temperature was 300 °C. Its formaldehyde conversion was higher than 90.0% at 400–600 °C. The synergistic effect between NiO and MoO3 guaranteed strong solid acidity, good redox properties and high concentration of surface oxygen species. It made NiO/MoO3 exhibit excellent hydrogen production performance. Furthermore, the in situ DRIFT study proved that the formaldehyde species adsorbed on the catalyst surface reacted with hydroxyl groups and water vapor also participated in the reaction. Then carbon monoxide (or carbon dioxide) and hydrogen formed by the decomposition of the intermediate. Finally, the crudely purified CNTs could be obtained by simple physical separation from the NiO/MoO3 catalyst and carbon deposits. The photosynthesis experiment also proved that the crudely purified CNTs could be used in the field of nanopesticides. They increased the plant yield by promoting plant photosynthesis.
中文翻译:

甲醛的蒸汽重整以产生氢气,并共同生产碳纳米管以增强光合作用
镍基催化剂表现出高催化活性,但是在蒸汽重整领域中会发生碳沉积(焦炭中毒)。为了解决镍基催化剂的焦炭中毒问题,将NiO / MoO 3催化剂用于甲醛蒸汽重整反应,并探索性地使用了共同生产的碳纳米管(CNT)来改善植物的光合作用。结果表明,NiO / MoO 3催化剂在600°C时具有最高的H 2选择性104.2%,其起始温度为300°C。在400–600°C下,其甲醛转化率高于90.0%。NiO和MoO 3的协同作用确保了较强的固体酸度,良好的氧化还原特性和高浓度的表面氧。制成NiO / MoO3显示出优异的制氢性能。此外,原位DRIFT研究证明,吸附在催化剂表面的甲醛与羟基发生了反应,水蒸气也参与了反应。然后一氧化碳(或二氧化碳)与氢气通过中间体分解而形成。最后,可以通过简单的物理分离从NiO / MoO 3催化剂和碳沉积物中获得粗纯化的CNT。光合作用实验也证明了粗纯化的碳纳米管可用于纳米农药领域。他们通过促进植物的光合作用提高了植物的产量。
更新日期:2020-07-06
中文翻译:

甲醛的蒸汽重整以产生氢气,并共同生产碳纳米管以增强光合作用
镍基催化剂表现出高催化活性,但是在蒸汽重整领域中会发生碳沉积(焦炭中毒)。为了解决镍基催化剂的焦炭中毒问题,将NiO / MoO 3催化剂用于甲醛蒸汽重整反应,并探索性地使用了共同生产的碳纳米管(CNT)来改善植物的光合作用。结果表明,NiO / MoO 3催化剂在600°C时具有最高的H 2选择性104.2%,其起始温度为300°C。在400–600°C下,其甲醛转化率高于90.0%。NiO和MoO 3的协同作用确保了较强的固体酸度,良好的氧化还原特性和高浓度的表面氧。制成NiO / MoO3显示出优异的制氢性能。此外,原位DRIFT研究证明,吸附在催化剂表面的甲醛与羟基发生了反应,水蒸气也参与了反应。然后一氧化碳(或二氧化碳)与氢气通过中间体分解而形成。最后,可以通过简单的物理分离从NiO / MoO 3催化剂和碳沉积物中获得粗纯化的CNT。光合作用实验也证明了粗纯化的碳纳米管可用于纳米农药领域。他们通过促进植物的光合作用提高了植物的产量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号