当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
First-Principles Investigation of the Thermal Degradation Mechanisms of Methylammonium Lead Triiodide Perovskite
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpcc.0c05123 Yi-Ching Chen, Wei-Jen Lin, Chih-Chiang Chiu, Ratna Juwita, Hui-Hsu Gavin Tsai
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jpcc.0c05123 Yi-Ching Chen, Wei-Jen Lin, Chih-Chiang Chiu, Ratna Juwita, Hui-Hsu Gavin Tsai
|
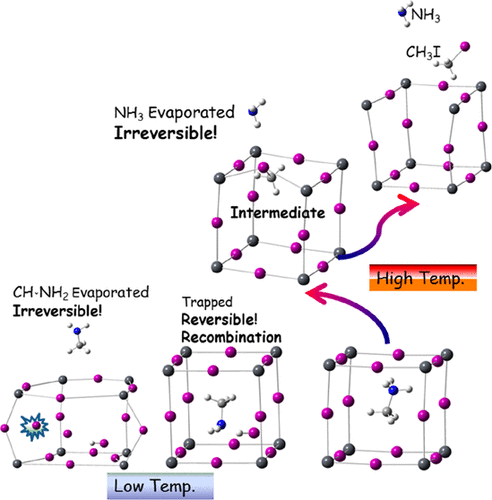
The poor stability of organometallic halide perovskites, especially CH3NH3PbI3 (MAPbI3), in high-temperature environments is one of the challenges retarding its wider applicability. A better understanding of how MAPbI3 perovskite degrades thermally will allow us to develop strategies to improve its “intrinsic” stability. In this study, we employed first-principles density functional theory to obtain detailed thermal degradation energy landscapes for MAPbI3. We focused on studying two reaction pathways: P1, for proton abstraction from methylammonium (MA) to iodine, and P2, for substitution (SN2) of an iodide anion (I–, as nucleophile) at the carbon atom of MA to yield iodomethane (CH3I) and ammonia (NH3). Our calculations provided kinetic data, allowing us to assess the “intrinsic” (in)stability of MAPbI3 under heat stress and to make sense of the seemingly contradictory experimental results. The I– species is more reactive for reaction P1 but not for P2. The energy of the reaction along pathway P1 increases monotonically with respect to proton abstraction. The reaction along pathway P2 is a two-step process, with a CH3–I–Pb–X intermediate formed in the crystal in conjunction with the release of NH3 gas. Our results suggest that the method of preparation (e.g., iodine-deficient conditions) and the morphology (e.g., improved crystallinity) of MAPbI3 could both influence its thermal stability.
中文翻译:

甲基铵三碘化铅钙钛矿热降解机理的第一性原理研究
有机金属卤化物钙钛矿的稳定性差,特别是CH 3 NH 3碘化铅3(MAPbI 3),在高温环境下是延迟它的更广泛的应用的挑战之一。更好地了解MAPbI 3钙钛矿如何热降解,将使我们能够制定改善其“固有”稳定性的策略。在这项研究中,我们采用第一原理密度泛函理论来获得MAPbI 3的详细热降解能分布图。我们专注于研究两种反应途径:P1,用于从甲基铵(MA)到碘的质子提取;P2,用于替代(S N2)碘阴离子(第I - ,作为亲核试剂)的MA的碳原子以产生碘甲烷(CH 3 NH I)和氨(3)。我们的计算提供了动力学数据,使我们能够评估热应激下MAPbI 3的“内在”(不稳定性),并理解似乎矛盾的实验结果。I –对反应P1更具反应性,但对P2则没有。沿着路径P1的反应能量相对于质子提取单调增加。沿着途径P2的反应是两步过程,其中CH 3与NH 3气体的释放一起在晶体中形成了–I–Pb–X中间体。我们的结果表明,MAPbI 3的制备方法(例如,缺碘条件)和形态(例如,改善的结晶度)都可能影响其热稳定性。
更新日期:2020-07-09
中文翻译:

甲基铵三碘化铅钙钛矿热降解机理的第一性原理研究
有机金属卤化物钙钛矿的稳定性差,特别是CH 3 NH 3碘化铅3(MAPbI 3),在高温环境下是延迟它的更广泛的应用的挑战之一。更好地了解MAPbI 3钙钛矿如何热降解,将使我们能够制定改善其“固有”稳定性的策略。在这项研究中,我们采用第一原理密度泛函理论来获得MAPbI 3的详细热降解能分布图。我们专注于研究两种反应途径:P1,用于从甲基铵(MA)到碘的质子提取;P2,用于替代(S N2)碘阴离子(第I - ,作为亲核试剂)的MA的碳原子以产生碘甲烷(CH 3 NH I)和氨(3)。我们的计算提供了动力学数据,使我们能够评估热应激下MAPbI 3的“内在”(不稳定性),并理解似乎矛盾的实验结果。I –对反应P1更具反应性,但对P2则没有。沿着路径P1的反应能量相对于质子提取单调增加。沿着途径P2的反应是两步过程,其中CH 3与NH 3气体的释放一起在晶体中形成了–I–Pb–X中间体。我们的结果表明,MAPbI 3的制备方法(例如,缺碘条件)和形态(例如,改善的结晶度)都可能影响其热稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号