当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unfolding Dynamics of a Photoswitchable Helical Peptide.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.jpcb.0c04017 Francois Auvray 1 , Jonathan D Hirst 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.jpcb.0c04017 Francois Auvray 1 , Jonathan D Hirst 1
Affiliation
|
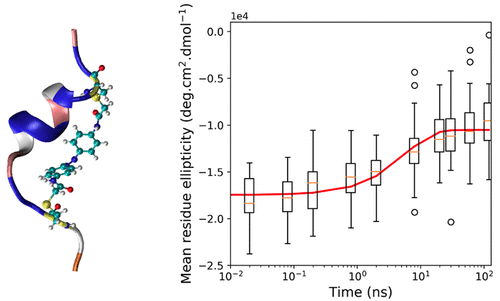
We present an atomistic force field for the azo-moiety of the photoswitchable FK-11-X peptide. We use the parameters to study the unfolding of the peptide through molecular dynamics simulations. The unfolded ensemble contains many different structures, ranging from a partially unfolded peptide to a fully unfolded structure. The averaged computed far-ultraviolet circular dichroism (CD) spectrum of the set of structures, which was simulated using the newly developed force field, agrees well with experiment. The rate of the simulated unfolding process was estimated to have a time constant of 5.80 ± 0.03 ns from the time evolution of the CD spectrum of the peptide, computed from the backbone conformations sampled over 40 simulated trajectories. Our estimated time constant is faster than, but not inconsistent with, previous experimental estimates from time-resolved infrared and optical rotatory dispersion spectroscopy.
中文翻译:

光开关螺旋肽的展开动力学。
我们提出了一个原子力场的光可切换的FK-11-X肽的偶氮部分。我们使用这些参数通过分子动力学模拟研究肽的展开。展开的集合包含许多不同的结构,范围从部分展开的肽到完全展开的结构。使用新开发的力场模拟的结构集的平均计算的远紫外圆二色性(CD)光谱与实验非常吻合。根据肽的CD光谱的时间演变估计,模拟的展开过程的速率具有5.80±0.03 ns的时间常数,该时间常数是根据在40条模拟轨迹上采样的骨架构象计算得出的。我们估算的时间常数要快于但并非不一致,
更新日期:2020-07-02
中文翻译:

光开关螺旋肽的展开动力学。
我们提出了一个原子力场的光可切换的FK-11-X肽的偶氮部分。我们使用这些参数通过分子动力学模拟研究肽的展开。展开的集合包含许多不同的结构,范围从部分展开的肽到完全展开的结构。使用新开发的力场模拟的结构集的平均计算的远紫外圆二色性(CD)光谱与实验非常吻合。根据肽的CD光谱的时间演变估计,模拟的展开过程的速率具有5.80±0.03 ns的时间常数,该时间常数是根据在40条模拟轨迹上采样的骨架构象计算得出的。我们估算的时间常数要快于但并非不一致,


























 京公网安备 11010802027423号
京公网安备 11010802027423号