当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Islatravir Case Study for Enhanced Screening of Thermodynamically Stable Crystalline Anhydrate Phases in Pharmaceutical Process Development by Hot Melt Extrusion.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.molpharmaceut.0c00316 Daniel Skomski 1 , Richard J Varsolona 1 , Yongchao Su 2 , Jingtao Zhang 3 , Ryan Teller 2 , Seth P Forster 2 , Stephanie Elizabeth Barrett 1 , Wei Xu 2
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.molpharmaceut.0c00316 Daniel Skomski 1 , Richard J Varsolona 1 , Yongchao Su 2 , Jingtao Zhang 3 , Ryan Teller 2 , Seth P Forster 2 , Stephanie Elizabeth Barrett 1 , Wei Xu 2
Affiliation
|
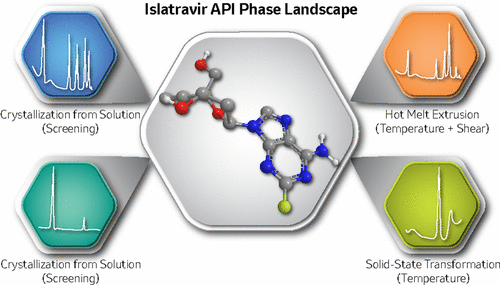
The emergence of new active pharmaceutical ingredient (API) polymorphs in pharmaceutical development presents significant risks. Even with thorough polymorph screening, new pathways toward alternate crystal phases can present themselves over the course of formulation development; thus, further improvements in phase screening methods are needed. Herein, a case study is presented of a thermodynamically stable crystalline phase of the HIV drug Islatravir (MK-8591, EFdA) that was not isolated from initial pharmaceutical polymorph screening. In total, five Islatravir phases are identified: one monohydrate and four anhydrate phases. The new phase, anhydrate form IV, was unexpectedly discovered during hot melt extrusion (HME) process development of polymeric implant drug product formulations while probing extreme manufacturing process conditions (elevated shear forces). X-ray diffraction (XRD), differential scanning calorimetry (DSC), and solid-state nuclear magnetic resonance (ssNMR) were utilized as principal tools to identify the new polymorph. The result suggests that HME introduces conditions that may allow a thermodynamically stable crystalline phase to form and these conditions are not necessarily captured by routine pharmaceutical polymorph screening. Subsequent investigations identified procedures to generate the new anhydrate phase without HME equipment by the use of special thermal procedures. It is found that for a crystalline hydrate phase the rate of water loss as well as water entrapment in a heating vessel play a crucial role in phase conversions into different anhydrate polymorphs. Further, the polymer involved in the HME manufacturing process also plays a critical role in the phase conversion, likely by coating the API microparticles and thereby altering the phase conversion kinetics. Strategies presented herein to mimic phase changes during formulation manufacture hold promise for the identification of thermodynamically stable anhydrate forms in earlier stages of pharmaceutical development.
中文翻译:

Islatravir案例研究,可通过热熔挤出技术增强制药工艺开发中热力学稳定的结晶无水物相的筛选。
在药物开发中新的活性药物成分(API)多晶型物的出现带来了重大风险。即使进行了彻底的多晶型物筛选,在配方开发过程中也会出现通往交替晶相的新途径。因此,需要进一步改进相位筛选方法。本文中,对未从初始药物多晶型物筛选中分离出的HIV药物Islatravir(MK-8591,EFdA)的热力学稳定结晶相进行了案例研究。总共鉴定出五个Islatravir相:一个一水合物相和四个无水相。新相,无水形式IV,在探索极端制造工艺条件(升高的剪切力)的同时,在聚合物植入药物产品配方的热熔挤出(HME)工艺开发过程中意外发现了这一现象。X射线衍射(XRD),差示扫描量热法(DSC)和固态核磁共振(ssNMR)被用作鉴定新多晶型物的主要工具。结果表明,HME引入了可能允许形成热力学稳定的结晶相的条件,而这些条件并不一定通过常规的药物多晶型物筛选来捕获。随后的调查确定了通过使用特殊的加热程序在不使用HME设备的情况下生成新的无水物相的程序。发现对于结晶水合物相,失水率以及在加热容器中的水截留在相转化为不同的无水多晶型物方面起关键作用。此外,参与HME制造过程的聚合物在相转化中也起着关键作用,可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。
更新日期:2020-08-03
中文翻译:

Islatravir案例研究,可通过热熔挤出技术增强制药工艺开发中热力学稳定的结晶无水物相的筛选。
在药物开发中新的活性药物成分(API)多晶型物的出现带来了重大风险。即使进行了彻底的多晶型物筛选,在配方开发过程中也会出现通往交替晶相的新途径。因此,需要进一步改进相位筛选方法。本文中,对未从初始药物多晶型物筛选中分离出的HIV药物Islatravir(MK-8591,EFdA)的热力学稳定结晶相进行了案例研究。总共鉴定出五个Islatravir相:一个一水合物相和四个无水相。新相,无水形式IV,在探索极端制造工艺条件(升高的剪切力)的同时,在聚合物植入药物产品配方的热熔挤出(HME)工艺开发过程中意外发现了这一现象。X射线衍射(XRD),差示扫描量热法(DSC)和固态核磁共振(ssNMR)被用作鉴定新多晶型物的主要工具。结果表明,HME引入了可能允许形成热力学稳定的结晶相的条件,而这些条件并不一定通过常规的药物多晶型物筛选来捕获。随后的调查确定了通过使用特殊的加热程序在不使用HME设备的情况下生成新的无水物相的程序。发现对于结晶水合物相,失水率以及在加热容器中的水截留在相转化为不同的无水多晶型物方面起关键作用。此外,参与HME制造过程的聚合物在相转化中也起着关键作用,可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。可能是通过涂覆API微粒从而改变相转化动力学来实现的。本文提出的在制剂制造过程中模拟相变的策略有望在药物开发的早期阶段鉴定热力学稳定的无水形式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号