Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Focal cerebral ischemia induces changes in oligodendrocytic tau isoforms in the damaged area.
Glia ( IF 6.2 ) Pub Date : 2020-06-09 , DOI: 10.1002/glia.23865 Mario Villa González 1, 2 , Laura Vallés-Saiz 2 , Ivó H Hernández 1, 2, 3 , Jesús Avila 2, 3 , Félix Hernández 2, 3, 4 , María José Pérez-Alvarez 1, 2, 3
Glia ( IF 6.2 ) Pub Date : 2020-06-09 , DOI: 10.1002/glia.23865 Mario Villa González 1, 2 , Laura Vallés-Saiz 2 , Ivó H Hernández 1, 2, 3 , Jesús Avila 2, 3 , Félix Hernández 2, 3, 4 , María José Pérez-Alvarez 1, 2, 3
Affiliation
|
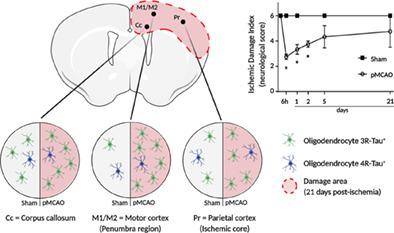
Ischemic stroke is a major cause of death and the first leading cause of long‐term disability worldwide. The only therapeutic strategy available to date is reperfusion and not all the patients are suitable for this treatment. Blood flow blockage or reduction leads to considerable brain damage, affecting both gray and white matter. The detrimental effects of ischemia have been studied extensively in the former but not in the latter. Previous reports indicate that preservation of white matter integrity reduces deleterious effect of ischemia on the brain. Oligodendrocytes are sensitive to ischemic damage, however, some reports demonstrate that oligodendrogenesis occurs after ischemia. These glial cells have a complex cytoskeletal network, including tau, that plays a key role to proper myelination. 4R‐Tau/3R‐Tau, which differ in the presence/absence of Exon 10, are found in oligodendrocytes; but the precise role of each isoform is not understood. Using permanent middle cerebral artery occlusion model and immunofluorescence, we demonstrate that cerebral ischemia induces an increase in 3R‐Tau versus 4R‐Tau in oligodendrocytes in the damaged area. In addition, cellular distribution of Tau undergoes a change after ischemia, with some oligodendrocytic processes showing positive staining for 3R‐Tau. This occurs simultaneously with the amelioration of neurological damage in ischemic rats. We propose that ischemia triggers an endogenous mechanism involving 3R‐Tau, that induces colonization of the ischemic damaged area by oligodendrocytes in an attempt to myelinate‐injured axons. Understanding the molecular mechanism of this phenomenon could pave the way for the design of therapeutic strategies that exploit glial cells for the treatment of ischemia.
中文翻译:

局灶性脑缺血诱导受损区域少突胶质细胞 tau 异构体的变化。
缺血性中风是导致死亡的主要原因,也是全球长期残疾的首要原因。迄今为止唯一可用的治疗策略是再灌注,并非所有患者都适合这种治疗。血流阻塞或减少会导致严重的脑损伤,影响灰质和白质。缺血的不利影响已在前者中得到广泛研究,但在后者中尚未得到广泛研究。以前的报告表明,白质完整性的保存减少了缺血对大脑的有害影响。少突胶质细胞对缺血性损伤敏感,然而,一些报告表明少突胶质细胞发生在缺血后。这些神经胶质细胞具有复杂的细胞骨架网络,包括 tau,它对正确的髓鞘形成起着关键作用。4R-Tau/3R-Tau,在少突胶质细胞中发现了外显子 10 存在/不存在的差异;但每种异构体的确切作用尚不清楚。使用永久性大脑中动脉闭塞模型和免疫荧光,我们证明脑缺血诱导受损区域少突胶质细胞中 3R-Tau 与 4R-Tau 的增加。此外,缺血后 Tau 的细胞分布发生变化,一些少突胶质细胞过程显示 3R-Tau 阳性染色。这与缺血大鼠神经损伤的改善同时发生。我们提出缺血触发了一种涉及 3R-Tau 的内源性机制,该机制诱导少突胶质细胞在缺血损伤区域定植,试图使受损的轴突髓鞘化。
更新日期:2020-06-09
中文翻译:

局灶性脑缺血诱导受损区域少突胶质细胞 tau 异构体的变化。
缺血性中风是导致死亡的主要原因,也是全球长期残疾的首要原因。迄今为止唯一可用的治疗策略是再灌注,并非所有患者都适合这种治疗。血流阻塞或减少会导致严重的脑损伤,影响灰质和白质。缺血的不利影响已在前者中得到广泛研究,但在后者中尚未得到广泛研究。以前的报告表明,白质完整性的保存减少了缺血对大脑的有害影响。少突胶质细胞对缺血性损伤敏感,然而,一些报告表明少突胶质细胞发生在缺血后。这些神经胶质细胞具有复杂的细胞骨架网络,包括 tau,它对正确的髓鞘形成起着关键作用。4R-Tau/3R-Tau,在少突胶质细胞中发现了外显子 10 存在/不存在的差异;但每种异构体的确切作用尚不清楚。使用永久性大脑中动脉闭塞模型和免疫荧光,我们证明脑缺血诱导受损区域少突胶质细胞中 3R-Tau 与 4R-Tau 的增加。此外,缺血后 Tau 的细胞分布发生变化,一些少突胶质细胞过程显示 3R-Tau 阳性染色。这与缺血大鼠神经损伤的改善同时发生。我们提出缺血触发了一种涉及 3R-Tau 的内源性机制,该机制诱导少突胶质细胞在缺血损伤区域定植,试图使受损的轴突髓鞘化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号