Cell Chemical Biology ( IF 8.6 ) Pub Date : 2020-06-09 , DOI: 10.1016/j.chembiol.2020.05.010 Mark Jelcic 1 , Ke Wang 2 , King Lam Hui 3 , Xiao-Chuan Cai 2 , Balázs Enyedi 4 , Minkui Luo 2 , Philipp Niethammer 3
|
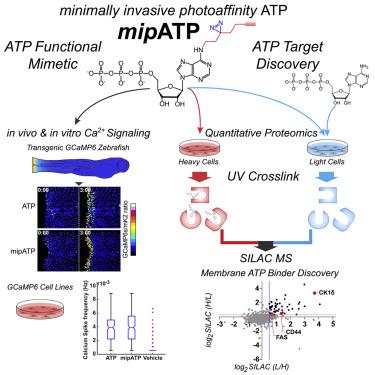
ATP is an important energy metabolite and allosteric signal in health and disease. ATP-interacting proteins, such as P2 receptors, control inflammation, cell death, migration, and wound healing. However, identification of allosteric ATP sites remains challenging, and our current inventory of ATP-controlled pathways is likely incomplete. Here, we develop and verify mipATP as a minimally invasive photoaffinity probe for ATP-interacting proteins. Its N6 functionalization allows target enrichment by UV crosslinking and conjugation to reporter tags by “click” chemistry. The additions are compact, allowing mipATP to completely retain the calcium signaling responses of native ATP in vitro and in vivo. mipATP specifically enriched for known nucleotide binders in A549 cell lysates and membrane fractions. In addition, it retrieved unannotated ATP interactors, such as the FAS receptor, CD44, and various SLC transporters. Thus, mipATP is a promising tool to identify allosteric ATP sites in the proteome.
中文翻译:

一种可点击照片的 ATP 模拟物揭示了膜蛋白质组中的核苷酸相互作用。
ATP 是健康和疾病中重要的能量代谢物和变构信号。ATP 相互作用蛋白,如 P2 受体,控制炎症、细胞死亡、迁移和伤口愈合。然而,变构 ATP 位点的识别仍然具有挑战性,我们目前的 ATP 控制途径清单可能不完整。在这里,我们开发并验证了mipATP作为 ATP 相互作用蛋白的微创光亲和探针。它的N 6功能化允许通过 UV 交联和通过“点击”化学与报告标签缀合来富集目标。添加是紧凑的,允许 mipATP在体外和体内完全保留天然 ATP 的钙信号反应. mipATP 特别富含 A549 细胞裂解物和膜组分中已知的核苷酸结合剂。此外,它还检索了未注释的 ATP 相互作用物,例如 FAS 受体、CD44 和各种 SLC 转运蛋白。因此,mipATP 是一种很有前途的工具,用于识别蛋白质组中的变构 ATP 位点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号