当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of polysubstituted 5-trifluoromethyl isoxazoles via denitrogenative cyclization of vinyl azides with trifluoroacetic anhydride
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0qo00243g Wei Wu 1, 2, 3, 4, 5 , Qiaoling Chen 1, 2, 3, 4, 5 , Yishi Tian 1, 2, 3, 4, 5 , Yihui Xu 1, 2, 3, 4, 5 , Yangjie Huang 1, 2, 3, 4, 5 , Yi You 1, 2, 3, 4, 5 , Zhiqiang Weng 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-06-08 , DOI: 10.1039/d0qo00243g Wei Wu 1, 2, 3, 4, 5 , Qiaoling Chen 1, 2, 3, 4, 5 , Yishi Tian 1, 2, 3, 4, 5 , Yihui Xu 1, 2, 3, 4, 5 , Yangjie Huang 1, 2, 3, 4, 5 , Yi You 1, 2, 3, 4, 5 , Zhiqiang Weng 1, 2, 3, 4, 5
Affiliation
|
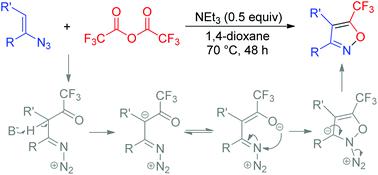
We describe a general method for the synthesis of 5-(trifluoromethyl)isoxazoles by denitrogenative cyclization of vinyl azides with trifluoroacetic anhydride in the presence of NEt3. The scope of this protocol was further extended to the synthesis of perfluoroalkylated isoxazoles. This reaction allows facile synthesis of structurally diverse 5-(perfluoroalkyl)isoxazole derivatives with various vinyl azides.
中文翻译:

乙烯基叠氮化物与三氟乙酸酐的脱氮环合反应合成多取代的5-三氟甲基异恶唑
我们描述了在NEt 3存在下,通过用三氟乙酸酐对叠氮化物进行脱氮环合反应来合成5-(三氟甲基)异恶唑的一般方法。该方案的范围进一步扩展到全氟烷基化异恶唑的合成。该反应使得可以容易地合成具有各种乙烯基叠氮化物的结构多样的5-(全氟烷基)异恶唑衍生物。
更新日期:2020-07-14
中文翻译:

乙烯基叠氮化物与三氟乙酸酐的脱氮环合反应合成多取代的5-三氟甲基异恶唑
我们描述了在NEt 3存在下,通过用三氟乙酸酐对叠氮化物进行脱氮环合反应来合成5-(三氟甲基)异恶唑的一般方法。该方案的范围进一步扩展到全氟烷基化异恶唑的合成。该反应使得可以容易地合成具有各种乙烯基叠氮化物的结构多样的5-(全氟烷基)异恶唑衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号