当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Biological Activity of 2,22-Dimethylene Analogues of 19-Norcalcitriol and Related Compounds.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jmedchem.0c00580 Izabela K Sibilska-Kaminski 1 , Rafal R Sicinski 2 , Lori A Plum 1 , Hector F DeLuca 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jmedchem.0c00580 Izabela K Sibilska-Kaminski 1 , Rafal R Sicinski 2 , Lori A Plum 1 , Hector F DeLuca 1
Affiliation
|
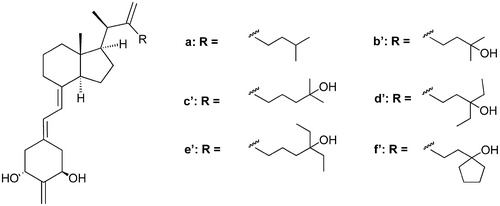
Continuing our search for vitamin D analogues, we explored the modification of the steroidal side chain and inserted a methylene moiety in position C-22 together with either lengthening the side chain or introducing a ring at the terminal end. Our conformational studies confirmed that the presence of a methylene group attached to C-22 restricts the conformational flexibility of the side chain, which can result in changes in biological characteristics of a molecule. All synthesized 1α,25-dihydroxy-2,22-dimethylene-19-norvitamin D3 analogues proved equal to calcitriol in their ability to bind to the vitamin D receptor, and most of them exert significantly higher differentiation and transcriptional activity than calcitriol. The most active compounds were characterized by the presence of an elongated side chain or 26,27-dimethylene bridge. The synthetic strategy was based on the Wittig–Horner coupling of the known A-ring phosphine oxide with the corresponding Grundmann ketones prepared from a 20-epi-Inhoffen-Lythgoe diol derived from vitamin D2.
中文翻译:

19-去甲三醇和相关化合物的2,22-二亚甲基类似物的合成和生物活性。
在继续寻找维生素D类似物的过程中,我们探索了甾体侧链的修饰方法,并在C-22位置插入了一个亚甲基部分,同时加长了侧链或在末端引入了环。我们的构象研究证实,连接至C-22的亚甲基的存在会限制侧链的构象柔韧性,从而导致分子生物学特性发生变化。所有合成的1α,25-二羟基-2,22-二亚甲基-19-正维生素D 3类似物在结合维生素D受体的能力方面被证明与骨化三醇相同,并且大多数类似物比骨化三醇具有更高的分化和转录活性。活性最高的化合物的特征是存在细长的侧链或26,27-二亚甲基桥。合成策略基于已知的A环氧化膦的Wittig-Horner偶联与相应的Grundmann酮的偶联,所述酮由从维生素D 2衍生的20-表位-Inhoffen-Lythgoe二醇制得。
更新日期:2020-07-09
中文翻译:

19-去甲三醇和相关化合物的2,22-二亚甲基类似物的合成和生物活性。
在继续寻找维生素D类似物的过程中,我们探索了甾体侧链的修饰方法,并在C-22位置插入了一个亚甲基部分,同时加长了侧链或在末端引入了环。我们的构象研究证实,连接至C-22的亚甲基的存在会限制侧链的构象柔韧性,从而导致分子生物学特性发生变化。所有合成的1α,25-二羟基-2,22-二亚甲基-19-正维生素D 3类似物在结合维生素D受体的能力方面被证明与骨化三醇相同,并且大多数类似物比骨化三醇具有更高的分化和转录活性。活性最高的化合物的特征是存在细长的侧链或26,27-二亚甲基桥。合成策略基于已知的A环氧化膦的Wittig-Horner偶联与相应的Grundmann酮的偶联,所述酮由从维生素D 2衍生的20-表位-Inhoffen-Lythgoe二醇制得。



























 京公网安备 11010802027423号
京公网安备 11010802027423号