当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal-Metalloligand Coordination Polymer Embedding Triangular Cobalt-Oxo Clusters: Solvent- and Temperature-Induced Crystal to Crystal Transformations and Associated Magnetism.
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.inorgchem.0c00762 Kun Fan 1 , Feng Xu 2, 3 , Mohamedally Kurmoo 4 , Xin-Da Huang 1 , Chwen-Haw Liao 1 , Song-Song Bao 1 , Fei Xue 2 , Li-Min Zheng 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.inorgchem.0c00762 Kun Fan 1 , Feng Xu 2, 3 , Mohamedally Kurmoo 4 , Xin-Da Huang 1 , Chwen-Haw Liao 1 , Song-Song Bao 1 , Fei Xue 2 , Li-Min Zheng 1
Affiliation
|
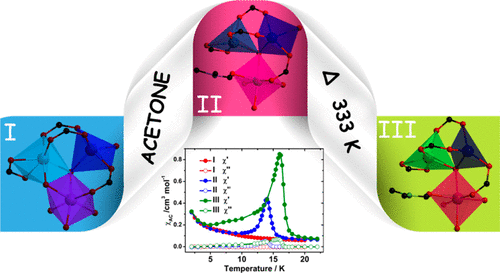
Reaction of the metalloligand IrIII(ppy-COOH)3 and the anisotropic paramagnetic CoII ion under solvothermal conditions resulted in a metal–metalloligand coordination polymer, [CoII3(μ3-O)(μ-OH2){IrIII(ppy-COO)2(ppy-COOH)}2(H2O)4]·2DMF·xH2O (I). It consists of trimeric Co3O secondary building units (SBUs) bridged by pairs of Ir to form chains of alternate orthogonal squares. The compound undergoes two single-crystal to single-crystal transformations while retaining its general structural features. A chemical transformation occurs to give [CoII3(μ3-O){IrIII(ppy-COO)2(ppy-COOH)}2(H2O)4(DMF)]·DMF·H2O (II) by soaking in acetone, where a bridging water molecule departs and the solvent DMF bonds to the vacant site of the Co center. Both I and II undergo a temperature-induced transformation to [CoII3(μ3-O){IrIII(ppy-COO)2(ppy-COOH)}2(H2O)3(DMF)]·DMF (III), where one more coordinated water molecule is lost. The major difference in the three phases is in the Co coordination spheres, which have considerable consequences on the magnetism. Compound I displays paramagnetism down to 2 K, whereas II and III show weak ferromagnetism with TC values of 14 and 17 K, respectively.
中文翻译:

嵌入三角形钴氧簇的金属-金属配位体配位聚合物:溶剂和温度诱导的晶体到晶体的转变和相关的磁性。
所述metalloligand铱的反应III(PPY-COOH)3和各向异性顺磁性钴II溶剂热的条件下离子导致金属metalloligand配位聚合物,[CO II 3(μ 3 -O)(μ-OH 2){铱III(ppy-COO)2(ppy-COOH)} 2(H 2 O)4 ]·2DMF· x H 2 O(I)。它由三聚体Co 3组成由一对Ir桥接的O个二级建筑单元(SBU),形成交替的正交正方形链。该化合物经历了两个单晶至单晶转变,同时保留了其一般结构特征。时,发生化学转化,得到[CO II 3(μ 3 -O){铱III(PPY-COO)2(PPY-COOH)} 2(H 2 O)4(DMF)]·DMF·H 2 O(II)浸泡在丙酮中,其中桥连的水分子离开,而溶剂DMF结合到Co中心的空位。无论我和II经受温度诱导转化为[有限公司II3(μ 3 -O){铱III(PPY-COO)2(PPY-COOH)} 2(H 2 O)3(DMF)]·DMF(III),其中,一个更加协调的水分子被丢失。三相的主要区别在于Co配位领域,这对磁性有很大影响。化合物I显示低至2 K的顺磁性,而化合物II和III显示T C分别为14 K和17 K的弱铁磁性。
更新日期:2020-07-06
中文翻译:

嵌入三角形钴氧簇的金属-金属配位体配位聚合物:溶剂和温度诱导的晶体到晶体的转变和相关的磁性。
所述metalloligand铱的反应III(PPY-COOH)3和各向异性顺磁性钴II溶剂热的条件下离子导致金属metalloligand配位聚合物,[CO II 3(μ 3 -O)(μ-OH 2){铱III(ppy-COO)2(ppy-COOH)} 2(H 2 O)4 ]·2DMF· x H 2 O(I)。它由三聚体Co 3组成由一对Ir桥接的O个二级建筑单元(SBU),形成交替的正交正方形链。该化合物经历了两个单晶至单晶转变,同时保留了其一般结构特征。时,发生化学转化,得到[CO II 3(μ 3 -O){铱III(PPY-COO)2(PPY-COOH)} 2(H 2 O)4(DMF)]·DMF·H 2 O(II)浸泡在丙酮中,其中桥连的水分子离开,而溶剂DMF结合到Co中心的空位。无论我和II经受温度诱导转化为[有限公司II3(μ 3 -O){铱III(PPY-COO)2(PPY-COOH)} 2(H 2 O)3(DMF)]·DMF(III),其中,一个更加协调的水分子被丢失。三相的主要区别在于Co配位领域,这对磁性有很大影响。化合物I显示低至2 K的顺磁性,而化合物II和III显示T C分别为14 K和17 K的弱铁磁性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号