当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structure of the SH3 domain of growth factor receptor-bound protein 2.
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-06-08 , DOI: 10.1107/s2053230x20007232 Alexandr Bolgov 1 , Svetlana Korban 1 , Dmitrii Luzik 2 , Vladimir Zhemkov 1 , Meewhi Kim 3 , Olga Rogacheva 2 , Ilya Bezprozvanny 1
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2020-06-08 , DOI: 10.1107/s2053230x20007232 Alexandr Bolgov 1 , Svetlana Korban 1 , Dmitrii Luzik 2 , Vladimir Zhemkov 1 , Meewhi Kim 3 , Olga Rogacheva 2 , Ilya Bezprozvanny 1
Affiliation
|
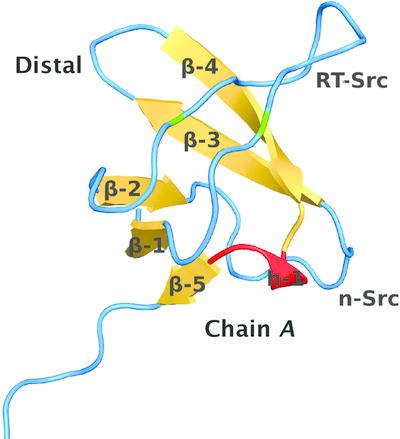
This study presents the crystal structure of the N‐terminal SH3 (SH3N) domain of growth factor receptor‐bound protein 2 (Grb2) at 2.5 Å resolution. Grb2 is a small (215‐amino‐acid) adaptor protein that is widely expressed and involved in signal transduction/cell communication. The crystal structure of full‐length Grb2 has previously been reported (PDB entry 1gri). The structure of the isolated SH3N domain is consistent with the full‐length structure. The structure of the isolated SH3N domain was solved at a higher resolution (2.5 Å compared with 3.1 Å for the previously deposited structure) and made it possible to resolve some of the loops that were missing in the full‐length structure. In addition, interactions between the carboxy‐terminal region of the SH3N domain and the Sos1‐binding sites were observed in the structure of the isolated domain. Analysis of these interactions provided new information about the ligand‐binding properties of the SH3N domain of Grb2.
中文翻译:

生长因子受体结合蛋白2的SH3结构域的晶体结构。
本研究以 2.5 Å 分辨率展示了生长因子受体结合蛋白 2 (Grb2) N 末端 SH3 (SH3N) 结构域的晶体结构。Grb2 是一种小型(215 个氨基酸)衔接蛋白,广泛表达并参与信号转导/细胞通讯。全长 Grb2 的晶体结构先前已被报道(PDB 条目 1gri)。分离的SH3N结构域的结构与全长结构一致。孤立的 SH3N 结构域的结构以更高分辨率解析(2.5 Å,而之前沉积的结构为 3.1 Å),并且可以解析全长结构中缺失的一些环。此外,在分离结构域的结构中观察到 SH3N 结构域的羧基末端区域与 Sos1 结合位点之间的相互作用。对这些相互作用的分析提供了有关 Grb2 SH3N 结构域的配体结合特性的新信息。
更新日期:2020-06-08
中文翻译:

生长因子受体结合蛋白2的SH3结构域的晶体结构。
本研究以 2.5 Å 分辨率展示了生长因子受体结合蛋白 2 (Grb2) N 末端 SH3 (SH3N) 结构域的晶体结构。Grb2 是一种小型(215 个氨基酸)衔接蛋白,广泛表达并参与信号转导/细胞通讯。全长 Grb2 的晶体结构先前已被报道(PDB 条目 1gri)。分离的SH3N结构域的结构与全长结构一致。孤立的 SH3N 结构域的结构以更高分辨率解析(2.5 Å,而之前沉积的结构为 3.1 Å),并且可以解析全长结构中缺失的一些环。此外,在分离结构域的结构中观察到 SH3N 结构域的羧基末端区域与 Sos1 结合位点之间的相互作用。对这些相互作用的分析提供了有关 Grb2 SH3N 结构域的配体结合特性的新信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号