当前位置:
X-MOL 学术
›
Synth. Met.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile approach to produce water-dispersible conducting polyaniline powder
Synthetic Metals ( IF 4.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.synthmet.2020.116451 Camila Aparecida Zimmermann , José Carlos Ferreira Júnior , Sílvia Daniela Araújo da Silva Ramôa , Guilherme Mariz de Oliveira Barra
Synthetic Metals ( IF 4.4 ) Pub Date : 2020-09-01 , DOI: 10.1016/j.synthmet.2020.116451 Camila Aparecida Zimmermann , José Carlos Ferreira Júnior , Sílvia Daniela Araújo da Silva Ramôa , Guilherme Mariz de Oliveira Barra
|
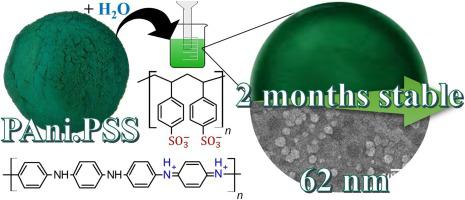
Abstract In this work, electrically conducting polyaniline (PAni) was obtained as a highly water-dispersible powder. The chemical aniline oxidative polymerization was conducted in the presence of poly(styrenesulfonic acid) (PSS) using ammonium peroxydisulfate (APS) as oxidative agent. The effects of aniline concentration and Ani:APS molar ratio on the redispersion capability of PAni.PSS powders were investigated. The purified PAni.PSS powders were redispersed in water and evaluated by UV–vis spectroscopy, field-emission scanning electron microscopy (FE-SEM) and dynamic light scattering (DLS). The electrical conductivity was measured on the dried material by the four-point probe method. As-prepared dispersions were also evaluated for comparative analyses. Conducting PAni.PSS as a powder with remarkable dispersibility and stability in water was obtained using Ani:APS 4:1 and aniline concentration of 0.256 mol.L−1. This material presented electrical conductivity of 10-3 S. cm−1 and granular morphology. Nanoparticles were obtained after sonication in water for only 4 min, with reproducible results. This colloidal dispersion remained stable for at least two months. Further analyses of zeta potential and stability in alkaline medium revealed negatively charged particles without flocculation signs in alkaline pHs for 240 h. At lower aniline concentration (0.032 mol.L−1) particles with 10 nm were obtained, however, not adequate for typical filtration. At Ani:APS 1:1 molar ratio yielded larger particles with heterogeneous distribution and partial precipitation.
中文翻译:

制备水分散性导电聚苯胺粉末的简便方法
摘要 在这项工作中,导电聚苯胺 (PAni) 是一种高度水分散性粉末。化学苯胺氧化聚合是在聚(苯乙烯磺酸)(PSS)存在下使用过二硫酸铵(APS)作为氧化剂进行的。研究了苯胺浓度和 Ani:APS 摩尔比对 PAni.PSS 粉末再分散能力的影响。将纯化的 PAni.PSS 粉末重新分散在水中,并通过紫外-可见光谱、场发射扫描电子显微镜 (FE-SEM) 和动态光散射 (DLS) 进行评估。通过四点探针法测量干燥材料的电导率。还评估了制备的分散体以进行比较分析。指挥帕尼。使用 Ani:APS 4:1 和 0.256 mol.L-1 的苯胺浓度获得了 PSS 作为粉末,在水中具有显着的分散性和稳定性。这种材料呈现 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。L-1。这种材料呈现出 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。L-1。这种材料呈现 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。
更新日期:2020-09-01
中文翻译:

制备水分散性导电聚苯胺粉末的简便方法
摘要 在这项工作中,导电聚苯胺 (PAni) 是一种高度水分散性粉末。化学苯胺氧化聚合是在聚(苯乙烯磺酸)(PSS)存在下使用过二硫酸铵(APS)作为氧化剂进行的。研究了苯胺浓度和 Ani:APS 摩尔比对 PAni.PSS 粉末再分散能力的影响。将纯化的 PAni.PSS 粉末重新分散在水中,并通过紫外-可见光谱、场发射扫描电子显微镜 (FE-SEM) 和动态光散射 (DLS) 进行评估。通过四点探针法测量干燥材料的电导率。还评估了制备的分散体以进行比较分析。指挥帕尼。使用 Ani:APS 4:1 和 0.256 mol.L-1 的苯胺浓度获得了 PSS 作为粉末,在水中具有显着的分散性和稳定性。这种材料呈现 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。L-1。这种材料呈现出 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。L-1。这种材料呈现 10-3 S. cm-1 的电导率和颗粒形态。在水中超声处理仅 4 分钟后获得纳米颗粒,结果可重复。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。这种胶态分散体保持稳定至少两个月。对碱性介质中的 zeta 电位和稳定性的进一步分析表明,在碱性 pH 值下 240 小时没有絮凝迹象的带负电粒子。然而,在较低的苯胺浓度 (0.032 mol.L-1) 下获得了 10 nm 的颗粒,这不足以用于典型的过滤。在 Ani:APS 1:1 的摩尔比下,产生具有不均匀分布和部分沉淀的较大颗粒。



























 京公网安备 11010802027423号
京公网安备 11010802027423号