Cell Calcium ( IF 4 ) Pub Date : 2020-06-08 , DOI: 10.1016/j.ceca.2020.102228 Lavanya Moparthi 1 , Satish Babu Moparthi 2 , Jérôme Wenger 3 , Peter M Zygmunt 4
|
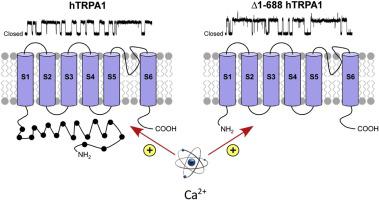
Extracellular influx of calcium or release of calcium from intracellular stores have been shown to activate mammalian TRPA1 as well as to sensitize and desensitize TRPA1 electrophilic activation. Calcium binding sites on both intracellular N- and C-termini have been proposed. Here, we demonstrate based on Förster resonance energy transfer (FRET) and bilayer patch-clamp studies, a direct calmodulin-independent action of calcium on the purified human TRPA1 (hTRPA1), causing structural changes and activation without immediate subsequent desensitization of hTRPA1 with and without its N-terminal ankyrin repeat domain (N-ARD). Thus, calcium alone activates hTRPA1 by a direct interaction with binding sites outside the N-ARD.
中文翻译:

在没有钙调蛋白的情况下,钙激活纯化的人 TRPA1,无论是否有其 N 端锚蛋白重复结构域。
细胞外钙的流入或细胞内钙的释放已被证明可以激活哺乳动物 TRPA1 以及使 TRPA1 亲电激活敏感和脱敏。已经提出了细胞内 N 端和 C 端的钙结合位点。在这里,我们证明了基于 Förster 共振能量转移 (FRET) 和双层膜片钳研究,钙对纯化的人 TRPA1 (hTRPA1) 的直接钙调素独立作用,导致结构变化和激活,而不会立即使 hTRPA1 脱敏 和没有其 N 端锚蛋白重复结构域 (N-ARD)。因此,单独的钙通过与 N-ARD 外结合位点的直接相互作用来激活 hTRPA1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号