Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-05-31 , DOI: 10.2174/1573406415666190626121650 Shaheen Faizi 1 , Tahira Sarfaraz 2 , Saima Sumbul 1 , Almas Jabeen 3 , Sobia A. Halim 3 , Mohammad A. Mesaik 3 , Zaheer Ul-Haq 3
|
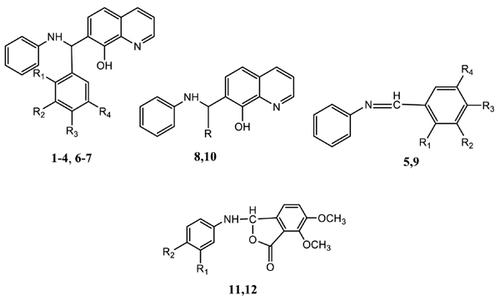
Background: In continuation of our work on Mannich reaction on 8-hydroxyquinoline, fifteen different combinations of aromatic aldehydes and aniline were subjected to Mannich reaction from which twelve products (eight Mannich bases, two imines and two intramolecularly cyclized products with benzofuranone skeleton) were obtained. Among them six compounds (1, 2, 6, 8, 9 and 12) are the new compounds. The structures of the compounds were characterized by UV, IR, MS and 1H NMR.
Methods: The compounds were tested for the inhibition of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β) at a concentration of 25 µg/mL. The cytokines were produced by THP-1 cells differentiated with PMA for 24hrs and stimulated with LPS for 4 hrs and supernatant were analyzed through ELISA technique.
Results and Discussion: Compounds 1-5, 8 and 9 inhibited the production of TNF-α and IL-1β. Compounds 1, 3, and 8 exerted potent inhibitions of TNF-α with 71%, 71%, and 83% inhibition, respectively. Compounds 1 and 8 significantly inhibited the production of IL-1β with 64% and 78% inhibition, respectively.
Conclusion: Compounds 1 and 8 significantly inhibited the production of IL-1β with 64% and 78% inhibition, respectively. Notably compound 8 showed the most potent inhibition of these cytokines. Additionally, the effect of compounds on viability of THP-1 cells was also evaluated. Moreover, molecular docking was carried out to study the mechanism of inhibition of TNF-α production.
中文翻译:

通过曼尼希反应合成新型8-羟基喹啉衍生物及其潜在的免疫调节剂生物学评价
背景:在继续我们对8-羟基喹啉进行曼尼希反应的工作中,对15种不同的芳香醛和苯胺组合进行了曼尼希反应,得到了十二种产物(八种曼尼希碱,两个亚胺和两个具有苯并呋喃酮骨架的分子内环化产物)。 。其中六个化合物(1、2、6、8、9和12)是新化合物。化合物的结构通过UV,IR,MS和1H NMR表征。
方法:以25 µg / mL的浓度测试这些化合物对促炎细胞因子肿瘤坏死因子-α(TNF-α)和白细胞介素-1β(IL-1β)的抑制作用。通过用PMA分化24小时并用LPS刺激4小时的THP-1细胞产生细胞因子,并通过ELISA技术分析上清液。
结果与讨论:化合物1-5、8和9抑制TNF-α和IL-1β的产生。化合物1、3和8分别有效抑制TNF-α,抑制作用分别为71%,71%和83%。化合物1和8分别以64%和78%抑制显着抑制IL-1β的产生。
结论:化合物1和8分别显着抑制IL-1β的产生,分别抑制64%和78%。值得注意的是,化合物8对这些细胞因子的抑制作用最强。另外,还评估了化合物对THP-1细胞活力的影响。此外,进行了分子对接研究了抑制TNF-α产生的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号