Medicinal Chemistry ( IF 2.3 ) Pub Date : 2020-05-31 , DOI: 10.2174/1573406415666190212105718 Muniza Shaikh 1 , Salman Siddiqui 2 , Humaira Zafar 1 , Uzma Naqeeb 2 , Fakiha Subzwari 1 , Rehan Imad 1 , Khalid M. Khan 2 , Muhammad I. Choudhary 1
|
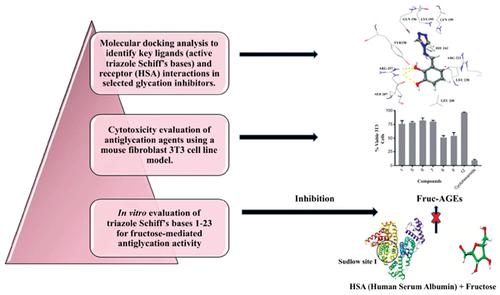
Background: Advanced glycation end products (AGEs) are known to be involved in the pathophysiology of diabetic complications, neurodegenerative diseases, and aging. Preventing the formation of AGEs can be helpful in the management of these diseases.
Objectives: Two classes of previously synthesized traizole Schiff’s bases (4H-1,2,4-triazole-4- Schiff’s bases 1-14, and 4H-1,2,4-triazole-3-Schiff’s bases 15-23) were evaluated for their in vitro antiglycation activity.
Methods: In vitro fructose-mediated human serum albumin (HSA) glycation assay was employed to assess the antiglycation activity of triazole Schiff’s bases. The active compounds were subjected to cytotoxicity analysis by MTT assay on mouse fibroblast (3T3) cell line. Molecular docking and simulation studies were carried out to evaluate the interactions and stability of compounds with HSA. Anti-hyperglycemic and antioxidant activities of selected non-cytotoxic compounds were evaluated by in vitro α-glucosidase inhibition, and DPPH free radical scavenging assays, respectively.
Results: Compound 1 (IC50=47.30±0.38 µM) from 4H-1,2,4-triazole-4-Schiff’s bases has exhibited antiglycation activity comparable to standard rutin (IC50=54.5±0.05 µM) along with a stable RMSD profile in MD simulation studies. Compound 1 also exhibited a potent α-glucosidase inhibitory activity, and moderate antioxidant property. Other derivatives showed a weak antiglycation activity with IC50 values between 248.1-637.7 µM. Compounds with potential antiglycation profile were found to be non-cytotoxic in a cellular assay.
Conclusion: The study identifies triazole Schiff’s bases active against fructose-mediated glycation of HSA, thus indicates their potential against late diabetic complications due to production of advancedend products (AGEs).
中文翻译:

三唑席夫氏碱对果糖介导的糖基化的抗糖化活性:体外和计算机模拟研究。
背景:已知晚期糖基化终产物(AGEs)与糖尿病并发症,神经退行性疾病和衰老的病理生理有关。预防AGEs的形成可能有助于控制这些疾病。
目的:评估了两类先前合成的曲唑席夫碱(4H-1,2,4-三唑-4-席夫碱1-14和4H-1,2,4-三唑-3-席夫碱15-23)。具有体外抗糖化活性
方法:采用果糖介导的人血清白蛋白(HSA)糖基化体外试验来评估三唑席夫碱的抗糖化活性。通过MTT测定法对小鼠成纤维细胞(3T3)细胞系上的活性化合物进行细胞毒性分析。进行了分子对接和模拟研究,以评估化合物与HSA的相互作用和稳定性。分别通过体外α-葡萄糖苷酶抑制作用和DPPH自由基清除试验评估了选定的非细胞毒性化合物的抗高血糖和抗氧化活性。
结果:来自4H-1,2,4-三唑-4-席夫碱的化合物1(IC50 = 47.30±0.38 µM)具有与标准芦丁(IC50 = 54.5±0.05 µM)相当的抗糖化活性,并在MD模拟研究。化合物1还显示出有效的α-葡糖苷酶抑制活性和适度的抗氧化性能。其他衍生物显示出较弱的抗糖化活性,IC50值为248.1-637.7 µM。在细胞测定法中发现具有潜在抗糖化作用的化合物无细胞毒性。
结论:该研究确定了三唑席夫氏碱对果糖介导的HSA糖基化具有活性,因此表明它们具有抗晚期糖尿病并发症(由于生产先进产品(AGEs))的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号