Letters in Organic Chemistry ( IF 0.8 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570178617666191203102528 Sayed K. Ramadan 1 , Wael S.I. Abou-Elmagd 1 , Ahmed I. Hashem 1
|
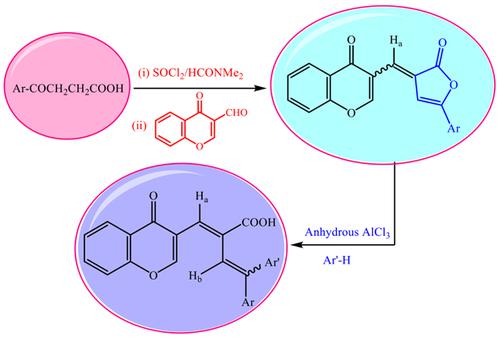
In this review, a survey on the behavior of 2(3H)-furanones as alkylating agents is systematized. It is obvious that the direction of the reaction was mainly dependent on the solvent used. Furanones reacted with AlCl3 in excess benzene, toluene, or anisole to give the corresponding butadienecarboxylic acids via an intermolecular alkylation mode. Carrying out the reaction in tetrachloroethane and nitrobenzene as solvents, the reactions may lead to intramolecular alkylation mode or fail to give any product and the unreacted furanones were isolated, depending upon the electron density on C-2 at the aryl group situated at position-3.
中文翻译:

2(3H)-呋喃酮的烷基化:分子间与分子内
在这篇综述中,对2(3H)-呋喃酮作为烷基化剂的行为进行了系统的调查。显然,反应的方向主要取决于所用的溶剂。呋喃酮与AlCl3在过量的苯,甲苯或苯甲醚中反应,通过分子间烷基化方式生成相应的丁二烯羧酸。在四氯乙烷和硝基苯作为溶剂中进行反应,该反应可能导致分子内烷基化模式或无法产生任何产物,并且未反应的呋喃酮被分离出来,具体取决于位于位置3的芳基上C-2上的电子密度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号