Letters in Drug Design & Discovery ( IF 1 ) Pub Date : 2020-05-01 , DOI: 10.2174/1570180816666190712102119 Hamidreza Akrami 1 , Bibi Fatemeh Mirjalili 2 , Omidreza Firuzi 3 , Azadeh Hekmat 4 , Ali Akbar Saboury 5 , Ramin Miri 3 , Omid Sabzevari 6 , Morteza Pirali-Hamedani 7 , Fereshteh Jeivad 6 , Setareh Moghimi 1 , Saeed Emami 8 , Alireza Foroumadi 7 , Mehdi Khoobi 1
|
Background: Chromene and anilinopyrimidine heterocyclics are attractive anticancer compounds that have inspired many researchers to design novel derivatives bearing improved anticancer activity.
Methods: A series of pyrimidine-fused benzo[f]chromene derivatives 6a-x were synthesized as anticancer hybrids of 1H-benzo[f]chromenes and anilinopyrimidines. The inhibitory activity of the synthesized compounds 6a-x against cell viability of human chronic myelogenous leukemia (K562), human acute lymphoblastic leukemia (MOLT-4) and human breast adenocarcinoma (MCF-7) cell lines was evaluated using MTT assay. The interaction of the most promising compound with calf-thymus DNA was also studied using spectrometric titrations and Circular Dichroism (CD) spectroscopy.
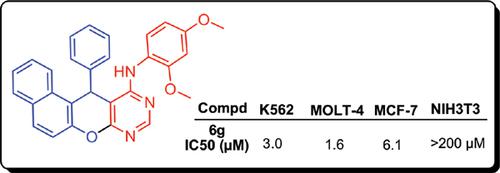
Results: Most compounds showed promising activity against tested cell lines. Among them, 2,4- dimethoxyanilino derivative 6g exhibited the best profile of activity against tested cell lines (IC50s = 1.6-6.1 μM) with no toxicity against NIH3T3 normal cell (IC50 >200 μM). The spectrometric studies exhibited that compound 6g binds to DNA strongly and may change DNA conformation significantly, presumably via a groove binding mechanism.
Conclusion: The results of this study suggest that the prototype compound 6g can be considered as a novel lead compound for the design and discovery of novel anticancer agents.
中文翻译:

新型氨基嘧啶衍生物的细胞毒活性和DNA结合特性
背景:Chromene和苯胺嘧啶杂环化合物是有吸引力的抗癌化合物,它激发了许多研究人员设计具有改善抗癌活性的新型衍生物。
方法:合成了一系列嘧啶稠合的苯并[f]苯甲基衍生物6a-x,作为1H-苯并[f]苯甲基和苯胺嘧啶的抗癌混合物。使用MTT分析评估了合成的化合物6a-x对人慢性骨髓性白血病(K562),人急性淋巴细胞性白血病(MOLT-4)和人乳腺腺癌(MCF-7)细胞系的抑制活性。还使用光谱滴定和圆二色谱(CD)光谱研究了最有希望的化合物与小牛胸腺DNA的相互作用。
结果:大多数化合物对测试的细胞系显示出有希望的活性。其中,2,4-二甲氧基苯胺基衍生物6g表现出对受试细胞系(IC50s = 1.6-6.1μM)的最佳活性,对NIH3T3正常细胞无毒性(IC50> 200μM)。光谱研究表明,化合物6g牢固地与DNA结合,并可能显着改变DNA构象,大概是通过凹槽结合机制。
结论:本研究结果表明,原型化合物6g可被视为设计和发现新型抗癌药物的新型先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号