当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regioselective C–F Bond Activation/C–C Bond Formation between Fluoropyridines and Cyclopropyl Groups at Zirconium
Organometallics ( IF 2.8 ) Pub Date : 2020-06-06 , DOI: 10.1021/acs.organomet.0c00218 Nuria Romero 1 , Quentin Dufrois 1 , Natalie Crespo 1 , Anthony Pujol 1 , Laure Vendier 1 , Michel Etienne 1
Organometallics ( IF 2.8 ) Pub Date : 2020-06-06 , DOI: 10.1021/acs.organomet.0c00218 Nuria Romero 1 , Quentin Dufrois 1 , Natalie Crespo 1 , Anthony Pujol 1 , Laure Vendier 1 , Michel Etienne 1
Affiliation
|
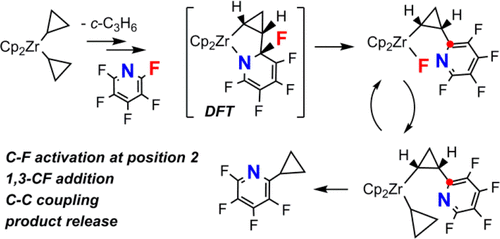
This paper addresses the problem of the strong and inert C–F bond activation of various fluoropyridines by zirconocene derivatives. Dicyclopropylzirconocene, [Cp2Zr(c-C3H5)2], thermally eliminates cyclopropane, giving the transient intermediate zirconabicyclobutane [Cp2Zr(η2-c-C3H4)] that cleaves a C–F bond of pentafluoropyridine selectively at position 2, forming new Zr–F and C–C bonds stereoselectively to give [Cp2ZrF{c-cis-CHCH2CH(2-NC5F4)}]. DFT computations indicate the selectivity results from the initial formation of an azazirconacycle intermediate that undergoes ring opening and C–F bond cleavage. Transmetalation with a variety of cyclopropyl donors yields [Cp2Zr(c-C3H5){c-cis-CHCH2CH(2-NC5F4)}] with the selectivity depending on the nature of the donor. A synthetic cycle is realized when [Cp2Zr(c-C3H5){c-cis-CHCH2CH(2-NC5F4)}] reacts with pentafluoropyridine, yielding 2-(c-C3H5)NC5F4 and [Cp2ZrF{c-cis-CHCH2CH(2-NC5F4)}] with C–F bond activation. Attempts at converting this reaction sequence to a catalytic version failed due to either decomposition of the active species or multiple C–F bond substitutions by the transmetalating agent.
中文翻译:

锆上的氟吡啶和环丙基之间的区域选择性C–F键激活/ CC键形成
本文探讨了锆茂衍生物对各种氟吡啶进行强而惰性的CF键活化的问题。Dicyclopropylzirconocene,混合[Cp 2 Zr的(ç -C 3 ħ 5)2 ],热消除环丙烷,给瞬时中间体zirconabicyclobutane的[Cp 2 Zr的(η 2 - ç -C 3 ħ 4)]裂解的C-F键在位置2选择性五氟,形成新ZRF和C-C键立体选择性,得到的[Cp 2 ZRF { ç -顺式- CHCH 2 CH(2-NC 5 ˚F4 }}]。DFT计算表明,选择性的结果是氮杂碳环中间体的初始形成,该中间体经历开环和CF键断裂。转移金属化与各种环丙基供体产量的[Cp 2 Zr的(ç -C 3 ħ 5){ Ç -顺式- CHCH 2 CH(2-NC 5 ˚F 4)}]具有取决于供体的性质的选择性。合成周期被实现时的[Cp 2 Zr的(ç -C 3 ħ 5){ Ç -顺式- CHCH 2 CH(2-NC 5˚F 4)}]发生反应与五氟,得到2-(ç -C 3 ħ 5)NC 5 ˚F 4和混合[Cp 2 ZRF { Ç -顺-CHCH 2(2-NC CH 5 ˚F 4)}]与C- F键活化。由于活性物质的分解或由重金属化剂进行的多个CF键取代,尝试将该反应序列转换为催化形式失败。
更新日期:2020-06-23
中文翻译:

锆上的氟吡啶和环丙基之间的区域选择性C–F键激活/ CC键形成
本文探讨了锆茂衍生物对各种氟吡啶进行强而惰性的CF键活化的问题。Dicyclopropylzirconocene,混合[Cp 2 Zr的(ç -C 3 ħ 5)2 ],热消除环丙烷,给瞬时中间体zirconabicyclobutane的[Cp 2 Zr的(η 2 - ç -C 3 ħ 4)]裂解的C-F键在位置2选择性五氟,形成新ZRF和C-C键立体选择性,得到的[Cp 2 ZRF { ç -顺式- CHCH 2 CH(2-NC 5 ˚F4 }}]。DFT计算表明,选择性的结果是氮杂碳环中间体的初始形成,该中间体经历开环和CF键断裂。转移金属化与各种环丙基供体产量的[Cp 2 Zr的(ç -C 3 ħ 5){ Ç -顺式- CHCH 2 CH(2-NC 5 ˚F 4)}]具有取决于供体的性质的选择性。合成周期被实现时的[Cp 2 Zr的(ç -C 3 ħ 5){ Ç -顺式- CHCH 2 CH(2-NC 5˚F 4)}]发生反应与五氟,得到2-(ç -C 3 ħ 5)NC 5 ˚F 4和混合[Cp 2 ZRF { Ç -顺-CHCH 2(2-NC CH 5 ˚F 4)}]与C- F键活化。由于活性物质的分解或由重金属化剂进行的多个CF键取代,尝试将该反应序列转换为催化形式失败。


























 京公网安备 11010802027423号
京公网安备 11010802027423号