当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fluorobenziodoxole-BF3 Reagent for Iodo(III)etherification of Alkynes in Ethereal Solvent.
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-06-07 , DOI: 10.1002/asia.202000653 Jinkui Chai 1, 2 , Wei Ding 1 , Junliang Wu 2 , Naohiko Yoshikai 1
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2020-06-07 , DOI: 10.1002/asia.202000653 Jinkui Chai 1, 2 , Wei Ding 1 , Junliang Wu 2 , Naohiko Yoshikai 1
Affiliation
|
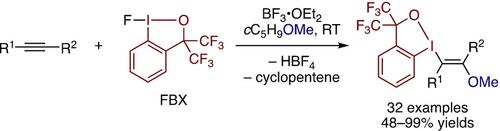
A combination of fluorobenziodoxole (FBX) and BF3 ⋅ OEt2 in cyclopentyl methyl ether promotes regio‐ and stereoselective addition of benziodoxole and methoxy groups to alkynes. This difunctionalization reaction tolerates a variety of functionalized internal and terminal alkynes to afford trans‐β‐alkoxyvinylbenziodoxoles, which represent versatile precursors to stereochemically well‐defined multisubstituted vinyl ethers. The reaction is proposed to involve cleavage of the I−F bond of FBX by BF3, followed by electrophilic activation of the alkyne by the resulting cationic IIII species that triggers the nucleophilic addition of the ethereal oxygen.
中文翻译:

氟代苯并恶唑-BF3试剂,用于在乙醇溶剂中炔烃的碘(III)醚化。
fluorobenziodoxole(FBX)的组合和BF 3 ⋅OET 2在环戊基甲基醚促进区域选择性和立体选择性加成benziodoxole和甲氧基与炔烃。这种双官能化反应可耐受各种官能化的内部和末端炔烃,以提供反式-β-烷氧基乙烯基苯并恶唑,这是立体化学定义明确的多取代乙烯基醚的多用途前体。提出该反应涉及通过BF 3裂解FBX的IF键,然后通过生成的阳离子I III物种引发炔的亲电活化,从而触发醚氧的亲核加成。
更新日期:2020-07-16
中文翻译:

氟代苯并恶唑-BF3试剂,用于在乙醇溶剂中炔烃的碘(III)醚化。
fluorobenziodoxole(FBX)的组合和BF 3 ⋅OET 2在环戊基甲基醚促进区域选择性和立体选择性加成benziodoxole和甲氧基与炔烃。这种双官能化反应可耐受各种官能化的内部和末端炔烃,以提供反式-β-烷氧基乙烯基苯并恶唑,这是立体化学定义明确的多取代乙烯基醚的多用途前体。提出该反应涉及通过BF 3裂解FBX的IF键,然后通过生成的阳离子I III物种引发炔的亲电活化,从而触发醚氧的亲核加成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号