Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2020-06-06 , DOI: 10.1016/j.apcata.2020.117693 J.P. Zhao , W.Y. Hernández , W.J. Zhou , Y. Yang , E.I. Vovk , M. Wu , N. Naghavi , M. Capron , V. Ordomsky
|
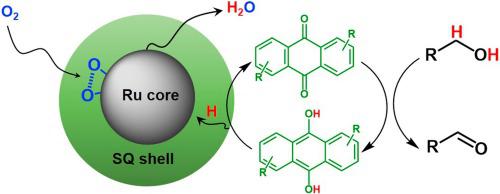
Selective aerobic oxidation of alcohols to corresponding carbonyl compounds is one of the most important challenges in the modern chemical industry. The existing metal based heterogeneous catalysts provide low selectivity due to over-oxidation of aldehydes to acids and esters. We have found that coating of Ru nanoparticles by disodium anthraquinone-2,6-disulfonate (SQ) results in selective oxidation of aliphatic, unsaturated and aromatic alcohols to aldehydes. Analysis of core-shell Ru@SQ catalyst shows strong interaction between Ru and SQ leading to change of their electronic state and structure. In-situ study of alcohol oxidation using FTIR and electrochemistry indicates on hydrogen abstraction by shell quinone species with hydrogen transfer by quinone to Ru core for water generation. Thus, the catalyst behavior mimics nano-electrocell by separation of oxidation reaction over quinone and reduction of oxygen over Ru providing higher selectivity to aldehyde.
中文翻译:

纳米单元Ru @醌酮核壳催化剂,用于将醇选择性氧化为羰基化合物
醇选择性好氧氧化为相应的羰基化合物是现代化学工业中最重要的挑战之一。现有的基于金属的非均相催化剂由于醛被过度氧化为酸和酯而提供了低选择性。我们发现用2,6-二磺酸蒽醌二钠(SQ)包覆Ru纳米颗粒会导致脂族,不饱和和芳族醇选择性氧化为醛。核-壳Ru @ SQ催化剂的分析表明Ru和SQ之间有很强的相互作用,从而导致它们的电子态和结构发生变化。原位FTIR和电化学法对醇氧化的研究表明,壳醌会吸收氢,醌将氢转移到Ru核中以生成水。因此,催化剂行为通过分离醌上的氧化反应和氧化钌上的氧而分离,从而模拟了纳米电化学电池,从而提供了对醛的更高选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号