当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of the chain length and metal : ligand ratio on the self-organization processes of Cu2+ complexes of [1 + 1] 1H-pyrazole azamacrocycles.
Dalton Transactions ( IF 4 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0dt01056a Alberto Lopera 1 , Ariadna Gil-Martínez , Javier Pitarch-Jarque , Begoña Verdejo , Salvador Blasco , M Paz Clares , Hermas R Jiménez , Enrique García-España
Dalton Transactions ( IF 4 ) Pub Date : 2020-06-05 , DOI: 10.1039/d0dt01056a Alberto Lopera 1 , Ariadna Gil-Martínez , Javier Pitarch-Jarque , Begoña Verdejo , Salvador Blasco , M Paz Clares , Hermas R Jiménez , Enrique García-España
Affiliation
|
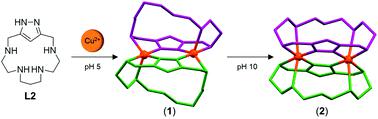
Three new [1 + 1] macrocycles formed by the reaction of 1H-3,5-bis(chloromethyl)pyrazole with the tosylated amines 1,4,7,10-tetraazadecane (L1), 1,4,8,11-tetraazaundecane (L2) and 1,5,10,14-tetraazatetradecane (L3) are described. Potentiometric studies and HR-ESI-Mass spectrometry show the formation of dimeric binuclear Cu2+ complexes whose organization depends on the type of hydrocarbon chains connecting the amine groups. Furthermore, trinuclear or/and tetranuclear complexes are formed depending also on the length of the polyaminic bridge and on the sequence of the hydrocarbon chains. The crystal structures of the [2 + 2] [Cu2(H(H−1L2))2](ClO4)4·4H2O (1) and [Cu2(H−1L2)2](ClO4)2 (2) complexes show in both of them two macrocycles self-assembled by the metal ions which interconnect their pyrazolate fragments that behave as bis(monodentate) ligands. While in 1 one central amine of each macrocycle binds to the axial position of a distorted square-pyramid and the other ones remain protonated, in 2 all the amine groups are involved in the coordination giving rise to a strongly distorted octahedral geometry. Paramagnetic 1H NMR measurements support that these structures also form in solution. Interestingly, tetranuclear complexes [Cu4(H−1L4)2(OH)2.08](ClO4)2.92Br0.54Cl0.46 (3) and [Pd2.39Cu1.61(H−1L4)2(OH)2](ClO4)2Cl1.33Br0.67·2.87H2O (4) have been isolated for the macrocycle containing the 1,5,9,13-tetraamine chain (L4). 3 has two binucleating units, one of them formed by the pyrazolate moieties and their neighbouring secondary amines and the other by the two central amines of both macrocycles. This latter Cu2+ coordination site is completed by two hydroxide anions as bridging ligands. 4 was obtained from a solution prepared to achieve full formation of the dimeric cage [Cu2(H−1(HL4))2]4+ by addition of K2PdCl4. The Pd2+ ion due to its softer acidic characteristics displaces the Cu2+ ions from the pyrazolate site. UV-vis spectroscopy suggests that the exchange is completed at room temperature after one hour.
中文翻译:

链长和金属:配体比对[1 +1] 1H-吡唑氮杂大环化合物Cu2 +配合物自组织过程的影响。
1 H -3,5-双(氯甲基)吡唑与甲苯磺酸胺1,4,7,10-四氮杂can (L1),1,4,8,11-的反应形成三个新的[1 +1]大环描述了四氮杂十一烷(L2)和1,5,10,14-四氮杂十八烷(L3)。电位测量研究和HR-ESI-质谱研究表明,二聚双核Cu 2+络合物的形成取决于其连接胺基的烃链类型。此外,还取决于聚酰胺桥的长度和烃链的序列形成三核或/和四核配合物。[2 + 2] [Cu 2(H(H -1 L2))的晶体结构2 ](ClO 4) 4 ·4H 2 O( 1)和[Cu 2(H -1 L2) 2 ](ClO 4) 2( 2)络合物在它们两个中均显示出两个大环,它们通过金属离子自组装,将它们的吡唑酸酯片段相互连接,使其表现为双(单齿)配体。在1中,每个大环的一个中心胺与变形的方形金字塔的轴向位置结合,而其他大环保持质子化,而在2中,所有胺基均参与配位,从而产生严重变形的八面体几何形状。顺磁性11 H NMR测量结果证明这些结构也在溶液中形成。有趣的是,四核配合物[Cu 4(H -1 L4)2(OH)2.08 ](ClO 4)2.92 Br 0.54 Cl 0.46(3)和[Pd 2.39 Cu 1.61(H -1 L4)2(OH)2 ]] [ ClO 4)2 Cl 1.33 Br 0.67 ·2.87H 2 O(4)已分离出含有1,5,9,13-四胺链(L4)。3具有两个双核化单元,其中一个由吡唑酸酯部分及其相邻的仲胺形成,另一个由两个大环的两个中心胺形成。后一个Cu 2+配位点由两个氢氧根阴离子作为桥连配体完成。4从溶液制备以实现完全形成二聚笼[铜,得到2(H -1(H L4))2 ] 4+通过加入的K 2的PdCl 4。Pd 2+离子由于其较软的酸性特性而取代了Cu 2+吡唑根部位的离子。紫外可见光谱法表明,该交换在室温下完成一小时。
更新日期:2020-06-29
中文翻译:

链长和金属:配体比对[1 +1] 1H-吡唑氮杂大环化合物Cu2 +配合物自组织过程的影响。
1 H -3,5-双(氯甲基)吡唑与甲苯磺酸胺1,4,7,10-四氮杂can (L1),1,4,8,11-的反应形成三个新的[1 +1]大环描述了四氮杂十一烷(L2)和1,5,10,14-四氮杂十八烷(L3)。电位测量研究和HR-ESI-质谱研究表明,二聚双核Cu 2+络合物的形成取决于其连接胺基的烃链类型。此外,还取决于聚酰胺桥的长度和烃链的序列形成三核或/和四核配合物。[2 + 2] [Cu 2(H(H -1 L2))的晶体结构2 ](ClO 4) 4 ·4H 2 O( 1)和[Cu 2(H -1 L2) 2 ](ClO 4) 2( 2)络合物在它们两个中均显示出两个大环,它们通过金属离子自组装,将它们的吡唑酸酯片段相互连接,使其表现为双(单齿)配体。在1中,每个大环的一个中心胺与变形的方形金字塔的轴向位置结合,而其他大环保持质子化,而在2中,所有胺基均参与配位,从而产生严重变形的八面体几何形状。顺磁性11 H NMR测量结果证明这些结构也在溶液中形成。有趣的是,四核配合物[Cu 4(H -1 L4)2(OH)2.08 ](ClO 4)2.92 Br 0.54 Cl 0.46(3)和[Pd 2.39 Cu 1.61(H -1 L4)2(OH)2 ]] [ ClO 4)2 Cl 1.33 Br 0.67 ·2.87H 2 O(4)已分离出含有1,5,9,13-四胺链(L4)。3具有两个双核化单元,其中一个由吡唑酸酯部分及其相邻的仲胺形成,另一个由两个大环的两个中心胺形成。后一个Cu 2+配位点由两个氢氧根阴离子作为桥连配体完成。4从溶液制备以实现完全形成二聚笼[铜,得到2(H -1(H L4))2 ] 4+通过加入的K 2的PdCl 4。Pd 2+离子由于其较软的酸性特性而取代了Cu 2+吡唑根部位的离子。紫外可见光谱法表明,该交换在室温下完成一小时。



























 京公网安备 11010802027423号
京公网安备 11010802027423号