当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lattice Oxygen Exchange in Rutile IrO2 during the Oxygen Evolution Reaction.
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jpclett.0c01258 Kevin Schweinar 1 , Baptiste Gault 1, 2 , Isabelle Mouton 1, 3 , Olga Kasian 4, 5, 6
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.jpclett.0c01258 Kevin Schweinar 1 , Baptiste Gault 1, 2 , Isabelle Mouton 1, 3 , Olga Kasian 4, 5, 6
Affiliation
|
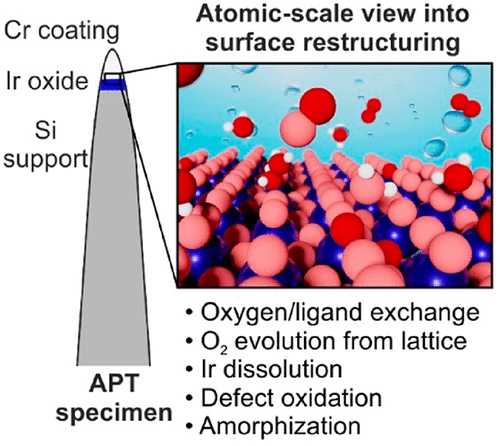
The development of efficient acidic water electrolyzers relies on understanding dynamic changes of the Ir-based catalytic surfaces during the oxygen evolution reaction (OER). Such changes include degradation, oxidation, and amorphization processes, each of which somehow affects the material’s catalytic performance and durability. Some mechanisms involve the release of oxygen atoms from the oxide’s lattice, the extent of which is determined by the structure of the catalyst. While the stability of hydrous Ir oxides suffers from the active participation of lattice oxygen atoms in the OER, rutile IrO2 is more stable and the lattice oxygen involvement is still under debate due to the insufficient sensitivity of commonly used online electrochemical mass spectrometry. Here, we revisit the case of rutile IrO2 at the atomic scale by a combination of isotope labeling and atom probe tomography and reveal the exchange of oxygen atoms between the oxide lattice and water. Our approach enables direct visualization of the electrochemically active volume of the catalysts and allows for the estimation of an oxygen exchange rate during the OER that is discussed in view of surface restructuring and subsequent degradation. Our work presents an unprecedented opportunity to quantitatively assess the exchange of surface species during an electrochemical reaction, relevant for the optimization of the long-term stability of catalytic systems.
中文翻译:

析氧反应过程中金红石型IrO2中的晶格氧交换
高效酸性水电解槽的开发依赖于了解氧释放反应(OER)过程中Ir基催化表面的动态变化。这些变化包括降解,氧化和非晶化过程,每一个都以某种方式影响材料的催化性能和耐久性。一些机理涉及氧原子从氧化物晶格中的释放,其程度取决于催化剂的结构。尽管含水的Ir氧化物的稳定性受到OER中晶格氧原子的主动参与的影响,但由于常用的在线电化学质谱法灵敏度不足,金红石IrO 2更加稳定,并且晶格氧的参与仍在争论中。在这里,我们回顾金红石型IrO的情况通过同位素标记和原子探针层析成像技术在原子尺度上观察到2,揭示了氧晶格与水之间的氧原子交换。我们的方法可以直接可视化催化剂的电化学活性体积,并可以估算OER期间的氧气交换速率,考虑到表面结构和随后的降解,对OER进行了讨论。我们的工作提供了前所未有的机会,可以定量评估电化学反应过程中表面物质的交换,这与优化催化系统的长期稳定性有关。
更新日期:2020-07-02
中文翻译:

析氧反应过程中金红石型IrO2中的晶格氧交换
高效酸性水电解槽的开发依赖于了解氧释放反应(OER)过程中Ir基催化表面的动态变化。这些变化包括降解,氧化和非晶化过程,每一个都以某种方式影响材料的催化性能和耐久性。一些机理涉及氧原子从氧化物晶格中的释放,其程度取决于催化剂的结构。尽管含水的Ir氧化物的稳定性受到OER中晶格氧原子的主动参与的影响,但由于常用的在线电化学质谱法灵敏度不足,金红石IrO 2更加稳定,并且晶格氧的参与仍在争论中。在这里,我们回顾金红石型IrO的情况通过同位素标记和原子探针层析成像技术在原子尺度上观察到2,揭示了氧晶格与水之间的氧原子交换。我们的方法可以直接可视化催化剂的电化学活性体积,并可以估算OER期间的氧气交换速率,考虑到表面结构和随后的降解,对OER进行了讨论。我们的工作提供了前所未有的机会,可以定量评估电化学反应过程中表面物质的交换,这与优化催化系统的长期稳定性有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号