当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Physiologically Based Pharmacokinetic Models Predicting Renal and Hepatic Concentrations of Industrial Chemicals after Virtual Oral Doses in Rats.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.chemrestox.0c00009 Yusuke Kamiya 1 , Shohei Otsuka 1 , Tomonori Miura 1 , Manae Yoshizawa 1 , Ayane Nakano 1 , Miyu Iwasaki 1 , Yui Kobayashi 1 , Makiko Shimizu 1 , Masato Kitajima 2 , Fumiaki Shono 3 , Kimito Funatsu 3 , Hiroshi Yamazaki 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-06-05 , DOI: 10.1021/acs.chemrestox.0c00009 Yusuke Kamiya 1 , Shohei Otsuka 1 , Tomonori Miura 1 , Manae Yoshizawa 1 , Ayane Nakano 1 , Miyu Iwasaki 1 , Yui Kobayashi 1 , Makiko Shimizu 1 , Masato Kitajima 2 , Fumiaki Shono 3 , Kimito Funatsu 3 , Hiroshi Yamazaki 1
Affiliation
|
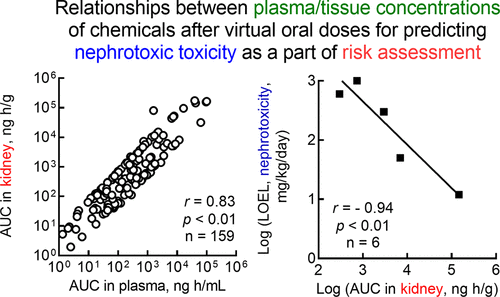
Recently developed high-throughput in vitro assays in combination with computational models could provide alternatives to animal testing. The purpose of the present study was to model the plasma, hepatic, and renal pharmacokinetics of approximately 150 structurally varied types of drugs, food components, and industrial chemicals after virtual external oral dosing in rats and to determine the relationship between the simulated internal concentrations in tissue/plasma and their lowest-observed-effect levels. The model parameters were based on rat plasma data from the literature and empirically determined pharmacokinetics measured after oral administrations to rats carried out to evaluate hepatotoxic or nephrotic potentials. To ensure that the analyzed substances exhibited a broad diversity of chemical structures, their structure-based location in the chemical space underwent projection onto a two-dimensional plane, as reported previously, using generative topographic mapping. A high-throughput in silico one-compartment model and a physiologically based pharmacokinetic (PBPK) model consisting of chemical receptor (gut), metabolizing (liver), central (main), and excreting (kidney) compartments were developed in parallel. For 159 disparate chemicals, the maximum plasma concentrations and the areas under the concentration–time curves obtained by one-compartment models and modified simple PBPK models were closely correlated. However, there were differences between the PBPK modeled and empirically obtained hepatic/renal concentrations and plasma maximal concentrations/areas under the concentration–time curves of the 159 chemicals. For a few compounds, the lowest-observed-effect levels were available for hepatotoxicity and nephrotoxicity in the Hazard Evaluation Support System Integrated Platform in Japan. The areas under the renal or hepatic concentration–time curves estimated using PBPK modeling were inversely associated with these lowest-observed-effect levels. Using PBPK forward dosimetry could provide the plasma/tissue concentrations of drugs and chemicals after oral dosing, thereby facilitating estimates of nephrotoxic or hepatotoxic potential as a part of the risk assessment.
中文翻译:

基于生理学的药代动力学模型预测大鼠虚拟口服给药后工业化学品的肾脏和肝脏浓度。
最近开发的高通量体外测定与计算模型相结合可以为动物试验提供替代方案。本研究的目的是在大鼠进行虚拟外部口服给药后,对大约150种结构不同类型的药物,食品成分和工业化学品的血浆,肝和肾药代动力学进行建模,并确定模拟体内浓度之间的关系。组织/血浆及其观察到的最低水平。模型参数基于文献中的大鼠血浆数据,并根据经验确定了对大鼠口服给药后的药代动力学,以评估其肝毒性或肾病潜能。为了确保所分析的物质具有广泛的化学结构差异,如先前报道,使用生成的地形图将它们在化学空间中的基于结构的位置投影到二维平面上。并行开发了一个高通量的计算机单室模型和一个基于生理的药代动力学(PBPK)模型,该模型由化学受体(肠),代谢(肝脏),中枢(主)和排泄(肾脏)室组成。对于159种不同的化学药品,一室模型和改进的简单PBPK模型获得的最大血浆浓度和浓度-时间曲线下的面积密切相关。但是,在159种化学物质的浓度-时间曲线下,PBPK模型和根据经验获得的肝/肾浓度和血浆最大浓度/面积之间存在差异。对于一些化合物,在日本的危害评估支持系统集成平台中,可观察到的肝毒性和肾毒性水平最低。使用PBPK模型估算的肾或肝浓度-时间曲线下的面积与这些最低的观察到的影响水平成反比。使用PBPK正向剂量测定法可以提供口服给药后药物和化学药品的血浆/组织浓度,从而有助于将肾毒性或肝毒性潜力作为风险评估的一部分进行估算。
更新日期:2020-07-20
中文翻译:

基于生理学的药代动力学模型预测大鼠虚拟口服给药后工业化学品的肾脏和肝脏浓度。
最近开发的高通量体外测定与计算模型相结合可以为动物试验提供替代方案。本研究的目的是在大鼠进行虚拟外部口服给药后,对大约150种结构不同类型的药物,食品成分和工业化学品的血浆,肝和肾药代动力学进行建模,并确定模拟体内浓度之间的关系。组织/血浆及其观察到的最低水平。模型参数基于文献中的大鼠血浆数据,并根据经验确定了对大鼠口服给药后的药代动力学,以评估其肝毒性或肾病潜能。为了确保所分析的物质具有广泛的化学结构差异,如先前报道,使用生成的地形图将它们在化学空间中的基于结构的位置投影到二维平面上。并行开发了一个高通量的计算机单室模型和一个基于生理的药代动力学(PBPK)模型,该模型由化学受体(肠),代谢(肝脏),中枢(主)和排泄(肾脏)室组成。对于159种不同的化学药品,一室模型和改进的简单PBPK模型获得的最大血浆浓度和浓度-时间曲线下的面积密切相关。但是,在159种化学物质的浓度-时间曲线下,PBPK模型和根据经验获得的肝/肾浓度和血浆最大浓度/面积之间存在差异。对于一些化合物,在日本的危害评估支持系统集成平台中,可观察到的肝毒性和肾毒性水平最低。使用PBPK模型估算的肾或肝浓度-时间曲线下的面积与这些最低的观察到的影响水平成反比。使用PBPK正向剂量测定法可以提供口服给药后药物和化学药品的血浆/组织浓度,从而有助于将肾毒性或肝毒性潜力作为风险评估的一部分进行估算。


























 京公网安备 11010802027423号
京公网安备 11010802027423号