当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular (directed) electrophilic C-H borylation.
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-06-04 , DOI: 10.1039/c9cs00763f S A Iqbal 1 , J Pahl 1 , K Yuan 1 , M J Ingleson 1
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-06-04 , DOI: 10.1039/c9cs00763f S A Iqbal 1 , J Pahl 1 , K Yuan 1 , M J Ingleson 1
Affiliation
|
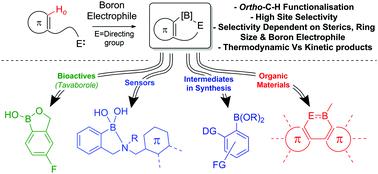
The intramolecular C–H borylation of (hetero)arenes and alkenes using electrophilic boranes is a powerful transition metal free methodology for forming C–B bonds. These C–H borylation reactions are preceded by intermolecular bond (both dative and covalent) formation, with examples proceeding via initial C–B and N–B bond formation dominating this field thus both are discussed in depth herein. Less prevalent intramolecular electrophilic C–H borylation reactions that proceed by intermolecular O–B, S–B and P–B bond formation are also summarised. Mechanistic studies are presented that reveal two mechanisms for C–H borylation, (i) electrophilic aromatic substitution (prevalent with B–X electrophiles); (ii) σ-bond metathesis mediated (prevalent with B–H and B–R electrophiles). To date, intramolecular electrophilic C–H borylation is utilised mainly for accessing boron containing conjugated organic materials, however recent developments, summarized herein alongside early studies, have highlighted the applicability of this methodology for forming synthetically versatile organo-boronate esters and boron containing bioactives. The multitude of synthetic procedures reported for intramolecular electrophilic C–H borylation contain many common features and this enables key requirements for successful C–H borylation and the factors effecting regioselectivity and substrate scope to be identified, discussed and summarized.
中文翻译:

分子内(定向)亲电CH硼酸酯化。
使用亲电硼烷对(杂)芳烃和烯烃进行分子内C–H硼化是一种强大的无过渡金属形成C–B键的方法。在这些C–H硼化反应之前,先形成分子间键(双向和共价),并通过最初的C–B和N–B键的形成主导了这一领域,因此,本文都将对其进行深入讨论。还总结了通过分子间OB,SB和PB键形成而发生的较不普遍的分子内亲电CH硼化反应。进行的机理研究揭示了C–H硼化的两种机理:(i)亲电芳族取代(普遍存在于B–X亲电体中);(ii)介导的σ键易位(普遍存在于BH和BR亲电体中)。迄今为止,分子内亲电C–H硼化主要用于获得含硼的共轭有机材料,然而,本文的近期进展以及早期研究总结了该方法在形成合成用途广泛的有机硼酸酯和含硼生物活性剂中的适用性。
更新日期:2020-07-06
中文翻译:

分子内(定向)亲电CH硼酸酯化。
使用亲电硼烷对(杂)芳烃和烯烃进行分子内C–H硼化是一种强大的无过渡金属形成C–B键的方法。在这些C–H硼化反应之前,先形成分子间键(双向和共价),并通过最初的C–B和N–B键的形成主导了这一领域,因此,本文都将对其进行深入讨论。还总结了通过分子间OB,SB和PB键形成而发生的较不普遍的分子内亲电CH硼化反应。进行的机理研究揭示了C–H硼化的两种机理:(i)亲电芳族取代(普遍存在于B–X亲电体中);(ii)介导的σ键易位(普遍存在于BH和BR亲电体中)。迄今为止,分子内亲电C–H硼化主要用于获得含硼的共轭有机材料,然而,本文的近期进展以及早期研究总结了该方法在形成合成用途广泛的有机硼酸酯和含硼生物活性剂中的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号