当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding Site Interactions of Modulators of Breast Cancer Resistance Protein, Multidrug Resistance-Associated Protein 2, and P-Glycoprotein Activity.
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.molpharmaceut.0c00155 Feng Deng 1 , Leo Ghemtio 1 , Evgeni Grazhdankin 2 , Peter Wipf 3 , Henri Xhaard 2 , Heidi Kidron 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.molpharmaceut.0c00155 Feng Deng 1 , Leo Ghemtio 1 , Evgeni Grazhdankin 2 , Peter Wipf 3 , Henri Xhaard 2 , Heidi Kidron 1
Affiliation
|
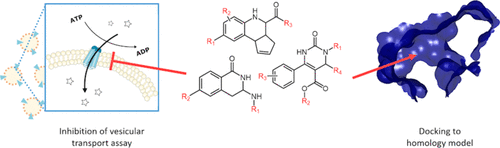
ATP-binding cassette (ABC)-transporters protect tissues by pumping their substrates out of the cells in many physiological barriers, such as the blood–brain barrier, intestine, liver, and kidney. These substrates include various endogenous metabolites, but, in addition, ABC transporters recognize a wide range of compounds, therefore affecting the disposition and elimination of clinically used drugs and their metabolites. Although numerous ABC-transporter inhibitors are known, the underlying mechanism of inhibition is not well characterized. The aim of this study is to deepen our understanding of transporter inhibition by studying the molecular basis of ligand recognition. In the current work, we compared the effect of 44 compounds on the active transport mediated by three ABC transporters: breast cancer resistance protein (BCRP and ABCG2), multidrug-resistance associated protein (MRP2 and ABCC2), and P-glycoprotein (P-gp and ABCB1). Eight compounds were strong inhibitors of all three transporters, while the activity of 36 compounds was transporter-specific. Of the tested compounds, 39, 25, and 11 were considered as strong inhibitors, while 1, 4, and 11 compounds were inactive against BCRP, MRP2, and P-gp, respectively. In addition, six transport-enhancing stimulators were observed for P-gp. In order to understand the observed selectivity, we compared the surface properties of binding cavities in the transporters and performed structure–activity analysis and computational docking of the compounds to known binding sites in the transmembrane domains and nucleotide-binding domains. Based on the results, the studied compounds are more likely to interact with the transmembrane domain than the nucleotide-binding domain. Additionally, the surface properties of the substrate binding site in the transmembrane domains of the three transporters were in line with the observed selectivity. Because of the high activity toward BCRP, we lacked the dynamic range needed to draw conclusions on favorable interactions; however, we identified amino acids in both P-gp and MRP2 that appear to be important for ligand recognition.
中文翻译:

乳腺癌耐药蛋白,多药耐药相关蛋白2和P-糖蛋白活性调节剂的结合位点相互作用。
ATP结合盒(ABC)转运蛋白通过将其底物从许多生理屏障(如血脑屏障,肠,肝和肾)中泵出细胞来保护组织。这些底物包括各种内源性代谢物,但此外,ABC转运蛋白可识别多种化合物,因此会影响临床使用的药物及其代谢物的处置和消除。尽管已知许多ABC-转运蛋白抑制剂,但其潜在的抑制机理尚不十分清楚。这项研究的目的是通过研究配体识别的分子基础来加深我们对转运蛋白抑制的理解。在目前的工作中,我们比较了44种化合物对三种ABC转运蛋白介导的主动转运的作用:乳腺癌抗性蛋白(BCRP和ABCG2),多药耐药相关蛋白(MRP2和ABCC2)和P-糖蛋白(P-gp和ABCB1)。八个化合物是所有三种转运蛋白的强抑制剂,而36种化合物的活性是转运蛋白特异性的。在测试的化合物中,有39、25和11被认为是强抑制剂,而1、4和11的化合物分别对BCRP,MRP2和P-gp没有活性。另外,观察到六个促进运输的刺激物的P-gp。为了了解观察到的选择性,我们比较了转运蛋白中结合腔的表面性质,并进行了结构活性分析以及化合物与跨膜结构域和核苷酸结合结构域中已知结合位点的计算对接。根据结果,研究的化合物比核苷酸结合结构域更可能与跨膜结构域相互作用。另外,在三个转运蛋白的跨膜结构域中底物结合位点的表面性质与观察到的选择性一致。由于对BCRP的活性很高,我们缺乏得出有利相互作用的结论所需要的动态范围。但是,我们在P-gp和MRP2中都发现了对配体识别很重要的氨基酸。
更新日期:2020-07-06
中文翻译:

乳腺癌耐药蛋白,多药耐药相关蛋白2和P-糖蛋白活性调节剂的结合位点相互作用。
ATP结合盒(ABC)转运蛋白通过将其底物从许多生理屏障(如血脑屏障,肠,肝和肾)中泵出细胞来保护组织。这些底物包括各种内源性代谢物,但此外,ABC转运蛋白可识别多种化合物,因此会影响临床使用的药物及其代谢物的处置和消除。尽管已知许多ABC-转运蛋白抑制剂,但其潜在的抑制机理尚不十分清楚。这项研究的目的是通过研究配体识别的分子基础来加深我们对转运蛋白抑制的理解。在目前的工作中,我们比较了44种化合物对三种ABC转运蛋白介导的主动转运的作用:乳腺癌抗性蛋白(BCRP和ABCG2),多药耐药相关蛋白(MRP2和ABCC2)和P-糖蛋白(P-gp和ABCB1)。八个化合物是所有三种转运蛋白的强抑制剂,而36种化合物的活性是转运蛋白特异性的。在测试的化合物中,有39、25和11被认为是强抑制剂,而1、4和11的化合物分别对BCRP,MRP2和P-gp没有活性。另外,观察到六个促进运输的刺激物的P-gp。为了了解观察到的选择性,我们比较了转运蛋白中结合腔的表面性质,并进行了结构活性分析以及化合物与跨膜结构域和核苷酸结合结构域中已知结合位点的计算对接。根据结果,研究的化合物比核苷酸结合结构域更可能与跨膜结构域相互作用。另外,在三个转运蛋白的跨膜结构域中底物结合位点的表面性质与观察到的选择性一致。由于对BCRP的活性很高,我们缺乏得出有利相互作用的结论所需要的动态范围。但是,我们在P-gp和MRP2中都发现了对配体识别很重要的氨基酸。


























 京公网安备 11010802027423号
京公网安备 11010802027423号