当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
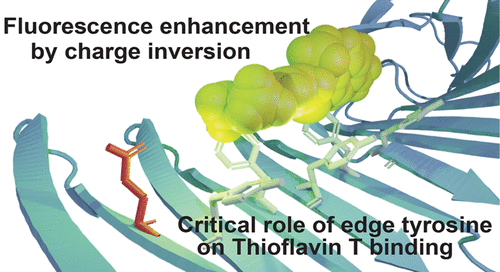
Residue-Specific Binding Mechanisms of Thioflavin T to a Surface of Flat β-Sheets within a Peptide Self-Assembly Mimic.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.biochem.0c00280 Sae Namioka 1 , Norio Yoshida 2 , Hiroyuki Konno 1 , Koki Makabe 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-04 , DOI: 10.1021/acs.biochem.0c00280 Sae Namioka 1 , Norio Yoshida 2 , Hiroyuki Konno 1 , Koki Makabe 1
Affiliation
|
Thioflavin T (ThT) is a popular fluorescent dye for detecting amyloid, a protein aggregate with a β-sheet-rich structure that causes many neurodegenerative diseases. Despite the dye’s popularity, a detailed understanding of its molecular binding mechanism remains elusive. We previously reported a protein model that can bind ThT on a single-layer β-sheet and revealed that a channel formed by aromatic rings with a confined length enhanced ThT binding. One of the mutants of the model system, 5-YY/LL, showed the highest affinity with a low micromolar dissociation constant. Here, we investigate the residue-specific mechanism of binding of ThT to 5-YY/LL. We introduced tyrosine to phenylalanine and tyrosine to histidine mutations into the channel. The mutants revealed that the fifth position of tyrosine (Y5) is important for binding of ThT. Positive charges introduced by histidine under a low-pH condition at the channel repel the binding of cationic ThT. Furthermore, we found a positive to negative conversion in the vicinity of the binding channel increases ThT fluorescence 4-fold. A detailed understanding of the ThT binding mechanism will enhance our ability to develop amyloid-specific small molecules.
中文翻译:

硫黄素T与肽自组装模拟物内平坦β-Sheets表面的残基特异性结合机制。
硫黄素T(ThT)是一种流行的荧光染料,用于检测淀粉样蛋白,淀粉样蛋白是一种富含β-折叠的结构的蛋白质聚集体,可引起许多神经退行性疾病。尽管该染料很受欢迎,但对其分子结合机理的详细了解仍然难以捉摸。我们先前报道了可以在单层β-折叠层上结合ThT的蛋白质模型,并揭示了由具有有限长度的芳香环形成的通道增强了ThT结合。模型系统的一种突变体5-YY / LL显示出最高的亲和力,且微摩尔解离常数低。在这里,我们研究了ThT与5-YY / LL结合的残基特异性机制。我们将酪氨酸引入苯丙氨酸,并将酪氨酸引入组氨酸突变进入通道。突变体显示酪氨酸的第五个位置(Y 5)对于绑定ThT很重要。组氨酸在低pH条件下在通道中引入的正电荷排斥阳离子ThT的结合。此外,我们发现在结合通道附近从正向负转换将ThT荧光提高了4倍。对ThT结合机制的详细了解将增强我们开发淀粉样蛋白特异性小分子的能力。
更新日期:2020-08-04
中文翻译:

硫黄素T与肽自组装模拟物内平坦β-Sheets表面的残基特异性结合机制。
硫黄素T(ThT)是一种流行的荧光染料,用于检测淀粉样蛋白,淀粉样蛋白是一种富含β-折叠的结构的蛋白质聚集体,可引起许多神经退行性疾病。尽管该染料很受欢迎,但对其分子结合机理的详细了解仍然难以捉摸。我们先前报道了可以在单层β-折叠层上结合ThT的蛋白质模型,并揭示了由具有有限长度的芳香环形成的通道增强了ThT结合。模型系统的一种突变体5-YY / LL显示出最高的亲和力,且微摩尔解离常数低。在这里,我们研究了ThT与5-YY / LL结合的残基特异性机制。我们将酪氨酸引入苯丙氨酸,并将酪氨酸引入组氨酸突变进入通道。突变体显示酪氨酸的第五个位置(Y 5)对于绑定ThT很重要。组氨酸在低pH条件下在通道中引入的正电荷排斥阳离子ThT的结合。此外,我们发现在结合通道附近从正向负转换将ThT荧光提高了4倍。对ThT结合机制的详细了解将增强我们开发淀粉样蛋白特异性小分子的能力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号