当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Metal Sequestration and Antimicrobial Activity of Human Calprotectin Are pH-Dependent.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.biochem.0c00359 Tomer Rosen 1 , Elizabeth M Nolan 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-03 , DOI: 10.1021/acs.biochem.0c00359 Tomer Rosen 1 , Elizabeth M Nolan 1
Affiliation
|
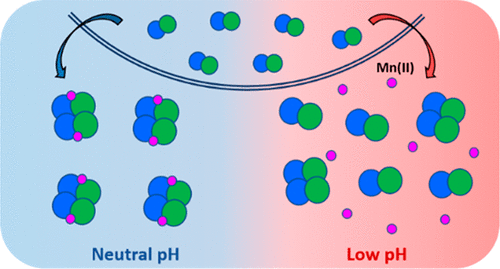
Human calprotectin (CP, S100A8/S100A9 oligomer) is an abundant innate immune protein that sequesters transition metal ions in the extracellular space to limit nutrient availability and the growth of invading microbial pathogens. Our current understanding of the metal-sequestering ability of CP is based on biochemical and functional studies performed at neutral or near-neutral pH. Nevertheless, CP can be present throughout the human body and is expressed at infection and inflammation sites that tend to be acidic. Here, we evaluate the metal binding and antimicrobial properties of CP in the pH range of 5.0–7.0. We show that Ca(II)-induced tetramerization, an important process for the extracellular functions of CP, is perturbed by acidic conditions. Moreover, a low pH impairs the antimicrobial activity of CP against some bacterial pathogens, including Staphylococcus aureus and Salmonella enterica serovar Typhimurium. At a mildly acidic pH, CP loses the ability to deplete Mn from microbial growth medium, indicating that Mn(II) sequestration is attenuated under acidic conditions. Evaluation of the Mn(II) binding properties of CP at pH 5.0–7.0 indicates that mildly acidic conditions decrease the Mn(II) binding affinity of the His6 site. Lastly, CP is less effective at preventing capture of Mn(II) by the bacterial solute-binding proteins MntC and PsaA at low pH. These results indicate that acidic conditions compromise the ability of CP to sequester Mn(II) and starve microbial pathogens of this nutrient. This work highlights the importance of considering the local pH of biological sites when describing the interplay between CP and microbes in host–pathogen interactions.
中文翻译:

人钙卫蛋白的金属螯合和抗菌活性是pH依赖性的。
人钙卫蛋白(CP,S100A8 / S100A9寡聚物)是一种丰富的先天免疫蛋白,可以隔离细胞外空间中的金属离子,从而限制营养物质的利用和入侵微生物病原体的生长。我们目前对CP的金属螯合能力的了解是基于在中性或接近中性pH值下进行的生化和功能研究。然而,CP可以存在于整个人体中,并在倾向于酸性的感染和炎症部位表达。在这里,我们评估了pH在5.0-7.0范围内CP的金属结合和抗菌性能。我们表明,Ca(II)诱导四聚,CP的细胞外功能的重要过程,被酸性条件扰动。此外,低pH值会削弱CP对某些细菌病原体的抗菌活性,金黄色葡萄球菌和肠沙门氏菌血清鼠伤寒。在弱酸性pH值下,CP失去了从微生物生长培养基中消耗Mn的能力,这表明Mn(II)的螯合在酸性条件下会减弱。在pH 5.0–7.0下对CP的Mn(II)结合特性的评估表明,弱酸性条件降低了His 6的Mn(II)结合亲和力现场。最后,在低pH下,CP不能有效防止细菌溶质结合蛋白MntC和PsaA捕获Mn(II)。这些结果表明酸性条件损害了CP螯合Mn(II)并使该营养物的微生物病原体饥饿的能力。这项工作强调了在描述CP和微生物在宿主与病原体相互作用中的相互作用时,考虑生物位点的局部pH的重要性。
更新日期:2020-07-07
中文翻译:

人钙卫蛋白的金属螯合和抗菌活性是pH依赖性的。
人钙卫蛋白(CP,S100A8 / S100A9寡聚物)是一种丰富的先天免疫蛋白,可以隔离细胞外空间中的金属离子,从而限制营养物质的利用和入侵微生物病原体的生长。我们目前对CP的金属螯合能力的了解是基于在中性或接近中性pH值下进行的生化和功能研究。然而,CP可以存在于整个人体中,并在倾向于酸性的感染和炎症部位表达。在这里,我们评估了pH在5.0-7.0范围内CP的金属结合和抗菌性能。我们表明,Ca(II)诱导四聚,CP的细胞外功能的重要过程,被酸性条件扰动。此外,低pH值会削弱CP对某些细菌病原体的抗菌活性,金黄色葡萄球菌和肠沙门氏菌血清鼠伤寒。在弱酸性pH值下,CP失去了从微生物生长培养基中消耗Mn的能力,这表明Mn(II)的螯合在酸性条件下会减弱。在pH 5.0–7.0下对CP的Mn(II)结合特性的评估表明,弱酸性条件降低了His 6的Mn(II)结合亲和力现场。最后,在低pH下,CP不能有效防止细菌溶质结合蛋白MntC和PsaA捕获Mn(II)。这些结果表明酸性条件损害了CP螯合Mn(II)并使该营养物的微生物病原体饥饿的能力。这项工作强调了在描述CP和微生物在宿主与病原体相互作用中的相互作用时,考虑生物位点的局部pH的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号